1-Aminoanthraquinone
Synonym(s):1-Aminoanthraquinone;Azoic Diazo No. 36;Fast Red AL
- CAS NO.:82-45-1
- Empirical Formula: C14H9NO2
- Molecular Weight: 223.23
- MDL number: MFCD00001213
- EINECS: 201-423-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-20 19:16:41
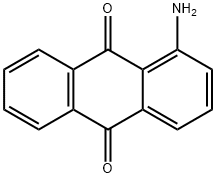
What is 1-Aminoanthraquinone?
Description
Anthraquinone dyes are the second largest class of dyes after azo dyes, and 1-aminoanthraquinone is an important intermediate for the synthesis of anthraquinone dyes. It is the main raw materials of amino acid and pyrazole anthraquinone occupy an extremely important position in the dye industry.
Chemical properties
deep brown crystalline powder. Soluble in hot nitrobenzene, toluene, xylene, ether, acetic acid, chloroform, benzene, slightly soluble in cold ethanol, insoluble in water.
The Uses of 1-Aminoanthraquinone
1-Aminoanthraquinone is the most important intermediate for manufacturing acid, reactive, disperse, and vat dyes.
What are the applications of Application
1-Aminoanthraquinone is a substituted anthraquinone
Preparation
1-Aminoanthraquinone (1-AAQ) is synthesized by the condensation of 2-substituted benzoic acid and xylene to yield 2-substituted-dimethylbenzophenone, subsequent oxidation of the methyl groups, ring closure to form a 1-substituted anthraquinone carboxylic acid, replacement of the 1-substituent with ammonia, and decarboxylation.
What are the applications of Application
1-Aminoanthraquinone has the ability to increase the solubility of anthraquinone derivatives in supercritical carbon dioxide. It is widely used in the preparation of various anthraquinone dyes. It also bridges Pt nanoparticles on carbon nanotubes as efficient electrocatalysts.
Hazard
Toxicity studies showed histopathological changes in the kidneys and spleen, loss of breastfeeding behaviour, decreased pup survival on day 4 after birth, repeated doses and reproductive toxicity less than 40 mg/kg/day (lowest tested dose) of NOAEL. No adverse effects on reproductive parameters or teratogenicity were observed. caused excitability, weight loss, and liver and kidney changes in an inhalation study in rats; orthocytotic anaemia, weight loss, and organ changes (liver, spleen and heart) in a 2-week oral study in guinea pigs; and was not irritating to the skin or eyes of rabbits.
Biochem/physiol Actions
1-Aminoanthraquinone has the ability to increase the solubility of derivatives of anthraquinone in supercritical carbon dioxide.
Safety Profile
Moderately toxic by intraperitoneal route. An eye irritant. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. When heated to decomposition it emits toxic NO,. See also AMINES
Synthesis
Anthraquinone is sulfonated with nicotinic sulfuric acid in the presence of a small amount of mercury salt to generate anthraquinone-1-sulfonic acid, which is then neutralized with potassium hydroxide to form anthraquinone sulfonic acid potassium salt. Under high temperature and high pressure, ammonia and anthraquinone sulfonic acid potassium The salt action generates 1-aminoanthraquinone, and the generated sulfite reacts with the product again and the quality of the product decreases. Therefore, nitrobenzene sulfonate is usually used as an oxidant to oxidize sulfite to sulfate, which itself is reduced to m-aminobenzenesulfonic acid.
Properties of 1-Aminoanthraquinone
| Melting point: | 253-255 °C (lit.) |
| Boiling point: | 364.52°C (rough estimate) |
| Density | 1.1814 (rough estimate) |
| refractive index | 1.4700 (estimate) |
| storage temp. | room temp |
| solubility | Soluble in alcohol, benzene, chlroform, ether, glacial acetic acid, HCl |
| form | powder to crystal |
| Colour Index | 37275 |
| pka | -0.51±0.20(Predicted) |
| color | Orange to Brown to Dark red |
| Water Solubility | 312.5ug/L(25 ºC) |
| Merck | 14,417 |
| BRN | 396360 |
| Stability: | Stable. Incompatible with acids, acid chlorides, acid anhydrides, chloroformates, strong oxidizing agents. |
| CAS DataBase Reference | 82-45-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Aminoanthraquinone(82-45-1) |
| EPA Substance Registry System | 1-Aminoanthraquinone (82-45-1) |
Safety information for 1-Aminoanthraquinone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H320:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. P391:Collect spillage. Hazardous to the aquatic environment P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for 1-Aminoanthraquinone
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 82-45-1 1-Aminoanthraquinone 98%View Details
82-45-1 1-Aminoanthraquinone 98%View Details
82-45-1 -
 1-Aminoanthraquinone CAS 82-45-1View Details
1-Aminoanthraquinone CAS 82-45-1View Details
82-45-1 -
 1-Aminoanthraquinone CAS 82-45-1View Details
1-Aminoanthraquinone CAS 82-45-1View Details
82-45-1 -
 1-Aminoanthraquinone CAS 82-45-1View Details
1-Aminoanthraquinone CAS 82-45-1View Details
82-45-1 -
 1-Aminoanthraquinone CAS 82-45-1View Details
1-Aminoanthraquinone CAS 82-45-1View Details
82-45-1 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
