1-Adamantyl Methacrylate
- CAS NO.:16887-36-8
- Empirical Formula: C14H20O2
- Molecular Weight: 220.31
- MDL number: MFCD08703330
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-03 18:37:10
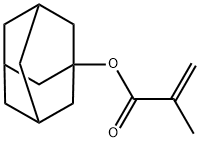
What is 1-Adamantyl Methacrylate?
Description
1-adamantyl methacrylate is a monomer that has a hydroxy group and a carbonyl group.
The Uses of 1-Adamantyl Methacrylate
1-adamantyl methacrylate is used in the production of polymers, as well as in coatings and adhesives. It can be thermally polymerized or crosslinked to form a polymer with high resistance to radiation and heat. This monomer has been used for the fabrication of organic light emitting diodes (OLEDs) and other device applications such as microelectronic circuits, solar cells, and displays. 1-Adamantyl Methacrylate can also be used in patterning materials using electron beam lithography or ultraviolet light exposure techniques. When exposed to radiation, this monomer undergoes a phase transition temperature from liquid to solid at about -30°C. The hydroxyl group attached to this monomer is also able to react with aliphatic or alicyclic compounds through palladium-catalyzed.
Preparation
The preparation of 1-adamantyl methacrylate is as follows:Stirrer, air inlet tube,Dean-Stark type water separator,Necked flask equipped with a stirrer, a thermometer,1-Adamantanol 100 g(0.657 mol),Methoquinone0.581 g,Hydroquinone0.143 g,Methacrylic acid227 g (2.64 mol) andMethylcyclohexaneWas added.After adding 1.61 g (0.0164 mol) of concentrated sulfuric acid,While blowing air at 10 mL / min, the mixture was stirred at a temperature of 100 to 115 ° C. for 4 hours under azeotropy of methylcyclohexane and water.4 hours laterThe conversion of 1-adamantanol was 95.1%.at this pointMethacrylic anhydride10.1 g (0.0657 mol / 2.0 equivalents to residual 1-adamantanol) was added,The mixture was further stirred for 1 hour,The reaction was terminated.The residual amount of 1-adamantanol after the reaction was 0.2%.The reaction solution was cooled to room temperature,313 g of a 20% by mass sodium hydroxide aqueous solution was added dropwise,The organic phase was washed.further,And washed twice with 100 mL of pure water.After distilling off the solvent with an evaporator, distillation under reduced pressure at 1 torr and 90 ° C.,122 g (yield 84.1%) of a colorless transparent liquid was obtained.This liquid was analyzed by GC and GPC.According to GC analysis1-Adamantyl acrylateThe purity of 99.5%.

What are the applications of Application
1-Adamantyl Methacrylate is a monomer that has a hydroxy group and a carbonyl group. It is used in the production of polymers, as well as in coatings and adhesives. 1-Adamantyl Methacrylate can be thermally polymerized or crosslinked to form a polymer with high resistance to radiation and heat.This monomer has been used for the fabrication of organic light emitting diodes (OLEDs) and other device applications such as microelectronic circuits, solar cells, and displays.
Research
1-Adamantyl methacrylate (AdMA) could be polymerized using the atom transfer radical polymerization (ATRP) method with methyl α-bromoisobutyrate (MBiB), copper(I) bromide (CuBr), copper(II) bromide (CuBr2) and 1,1,4,7,10,10-hexamethyltriethylenetetramine (HMTETA) in toluene at 60?°C, producing well-defined poly(1-adamantyl methacrylate) (PAdMA). The controlled/living radical polymerization of methacrylate monomers bearing bulky ester groups, such as AdMA, which guarantees the generation of well-defined macromolecular architectures such as the block copolymer, graft copolymer and star-shaped polymer on a preparative scale, is still of great interest[1].
References
[1] Keita Fuchise. “Precise synthesis of poly(1-adamantyl methacrylate) by atom transfer radical polymerization.” Polymer Journal 41 1 (2010): 626–631.
Properties of 1-Adamantyl Methacrylate
| Boiling point: | 289.3±9.0 °C(Predicted) |
| Density | 1.07±0.1 g/cm3(Predicted) |
| refractive index | 1.50 |
| storage temp. | 2-8°C |
| form | powder to lump to clear liquid |
| color | White or Colorless to Almost white or Almost colorless |
| InChI | InChI=1S/C14H20O2/c1-9(2)13(15)16-14-6-10-3-11(7-14)5-12(4-10)8-14/h10-12H,1,3-8H2,2H3 |
Safety information for 1-Adamantyl Methacrylate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1-Adamantyl Methacrylate
| InChIKey | MZVABYGYVXBZDP-UHFFFAOYSA-N |
| SMILES | C(OC12CC3CC(CC(C3)C1)C2)(=O)C(C)=C |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 1-Adamantyl Methacrylate (stabilized with MEHQ) CAS 16887-36-8View Details
1-Adamantyl Methacrylate (stabilized with MEHQ) CAS 16887-36-8View Details
16887-36-8 -
 Adamantan-1-yl methacrylate 95.00% CAS 16887-36-8View Details
Adamantan-1-yl methacrylate 95.00% CAS 16887-36-8View Details
16887-36-8 -
 Adamantan-1-yl methacrylate 98% (GC) CAS 16887-36-8View Details
Adamantan-1-yl methacrylate 98% (GC) CAS 16887-36-8View Details
16887-36-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1