(+)-MAGNOFLORINE IODIDE
- CAS NO.:2141-09-5
- Empirical Formula: C20H24INO4
- Molecular Weight: 469.31
- MDL number: MFCD03427294
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
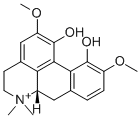
What is (+)-MAGNOFLORINE IODIDE?
Description
A wide variety of species contain this quaternary alkaloid which is widespread among the families, Aquilegia, Magnolia and Michelia. It has been isolated and characterized as the iodide, m.p. 248-9°C (dec.); [0:11>5 + 200.1° (MeOH). The optically inactive base also yields an iodide which crystallizes with 1.5 H20, m.p. 243°C (dec.).
Description
Magnoflorine is an alkaloid and a major constituent of S. acutum that has diverse biological activities. It reduces hemolysis induced by lysophosphatidylcholine (LPC), but not NaCl, in a rat erythrocyte suspension when used at concentrations ranging from 0.01 to 10 μM. Magnoflorine (10 μM) induces intracellular chloride influx in neuroblastoma cells, an effect that is reversed by the GABAA receptor antagonist bicuculline . It reduces TNF-α-induced NF-κB expression as well as production of IL-6 and IL-8 in BEAS-2B cells. In vivo, magnoflorine (20 mg/kg, i.p.) improves short- and long-term memory acquisition in a passive avoidance test in a mouse model of scopolamine-induced memory impairment.
The Uses of (+)-MAGNOFLORINE IODIDE
Magnoflorine-d3 is the labelled analogue of Magnoflorine, an isoquinoline alkaloid from the Coptis japonica and it enhances the myoblast differentiation through activation of the p38 MAP kinase and Akt pathway.
Definition
ChEBI: (S)-magnoflorine is an aporphine alkaloid that is (S)-corytuberine in which the nitrogen has been quaternised by an additional methyl group. It has a role as a plant metabolite. It is an aporphine alkaloid and a quaternary ammonium ion. It is functionally related to a (S)-corytuberine.
References
Nakano., Chem. Pharm. Bull. (Tokyo), 2, 329 (1954) Tomita, Kikkawa.,J. Pharm. Soc., Japan, 77, 195 (1957) Gopinath et aI., J. Sci. Ind. Res., 18B, 444 (1959) Ishii, Harada.,J. Pharm. Soc., Japan, 81,238 (1961) Yang, Lu, Hsiao., ibid, 82,794,816 (1962) Yang, Lu, Hsiao., ibid, 83,216 (1963)
Properties of (+)-MAGNOFLORINE IODIDE
| Melting point: | 252℃ |
| storage temp. | 2-8°C(protect from light) |
| solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. |
| form | Powder |
Safety information for (+)-MAGNOFLORINE IODIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for (+)-MAGNOFLORINE IODIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Magnoflorine CAS 2141-09-5View Details
Magnoflorine CAS 2141-09-5View Details
2141-09-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1