(-)-LUPININE
- CAS NO.:486-70-4
- Empirical Formula: C10H19NO
- Molecular Weight: 169.26
- MDL number: MFCD00213431
- EINECS: 207-638-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
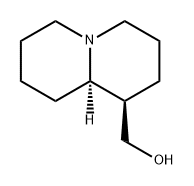
What is (-)-LUPININE?
Description
This lupin alkaloid was first obtained by Baumert and is present in numerous
plants of the Lupinus family, the most important sources being L. luteus, L.
niger and L. palmeri Wats. It crystallizes from light petroleum in colourless
rhombs and may be boiled without decomposition in a stream of hydrogen. It is
laevorotatory with [α]17D - 20.35° (EtOH) and is soluble in H20 and most
organic solvents but only sparingly so in petroleum ether. It is a sufficiently
strong base to liberate ammonia from its salts. The hydrochloride forms colourless rhombic crystals from aqueous EtOH, m.p. 2l2-3°C; [α]D - 14° (H20);
the hydriodide has m.p. 140-1 DC; aurichloride, m.p. 211-3°C; platinichloride
as yellow crystals, m.p. 166-166.5°C; (+)-tartrate, m.p. 171°C; [α]D + 15.5°
(EtOH); methochloride, m.p. 212-3°C; methiodide, m.p. 295-6°C; phenylurethane, m.p. 98-9°C; (-)-camphorsulphonate, m.p. 184°C; [α]D - 15.3° and the
(+)-camphorsulphonate, m.p. 182-3°C; [α]D + 22.5°. The formation of a
benzoyl derivative, m.p. 49-S00 C, oxidation of the alkaloid to lupininic acid,
C9H16.COOH, m.p. 255°C and dehydration to anhydrolupinine, ClOH17N, a
colourless oil of unpleasant odour, b.p. 216-7°C/726 mm all show the presence
of a primary alcoholic hydroxyl group. Lupinine does not contain a methylimino
group and behaves as a tertiary base. On exhaustive methylation it gives, in three
stages, trimethylamine and an unsaturated alcohol. From this, it may be deduced
that the alkaloid contains a bicyc1ic system. Several unsuccessful attempts were made to synthesize the alkaloid before this was finally achieved by Clemo and
his co-workers.
This alkaloid, like the others of the lupinane group, is of little importance in
medicine although the p-aminobenzoate has been shown to possess a marked
local anaesthetic action.
The (+ )-form of the alkaloid has m.p. 68°C; [α]D + 19.9° and yields the (-)-
tartrate, m.p.167-8°C; [α]D -15.8°.
Chemical properties
Crystalline solid, obtained as orthorhombic prisms from acetone; melts at 69°C(156.2°F); bp 270°C (518°F); strongly basic;soluble in water and organic solvents.
The Uses of (-)-LUPININE
(-)-Lupinine is an alkaloid capable of counteracting ethanol anesthesia.
The Uses of (-)-LUPININE
antifeedant, antiinflammatory, oxytoxic
The Uses of (-)-LUPININE
The ι-form of lupinine occurs in seeds andherb of Lupinus luteous L., Chenopodiaceae,and other lupinus species. Its clinical applications are very limited.
What are the applications of Application
(?)-Lupinine is an alkaloid capable of counteracting ethanol anesthesia
Definition
ChEBI: Lupinine is a quinolizidine alkaloid.
Health Hazard
This alkaloid is moderately toxic. The toxicaction, however, is lower than that of cytisine. Ingestion of high doses may producenausea, convulsions, and respiratory fail ure. The lethal dose in guinea pigs by theintraperitoneal route is 28 mg/kg..
References
Baumert., Ber., 14, 1150, 1321, 1880, 1882 (1881)
Baumert., ibid, 15,631,1951 (1882)
Schmidt, Berend., Arch. Pharm., 235,263 (1897)
Willstiitter, Fourneau., Ber., 35, 1914 (1902)
Karrer et al., Helv. Chirn. Acta, 11, 1062 (1928)
Clemo, Raper, J. Chern. Soc., 1927 (1929)
Winterfeldt, Cosel., Arch. Pharrn., 70, 278 (1940)
Sadykov, Spasokukotski., J. Gen. Chern. USSR, 13,830 (1943)
Sadykov., ibid, 19,143 (1949)
Zaboev., ibid, 18,194 (1948)
Ratusky, Sorm., Chern. Listy., 47, 1491 (1953)
Properties of (-)-LUPININE
| Melting point: | 62-65°C |
| Boiling point: | 160-164°C 4mm |
| alpha | D26 -25.9° (c = 3 in water); D28 -21° (c = 9.5 in alcohol) |
| Density | 0.9660 (rough estimate) |
| refractive index | 1.4610 (estimate) |
| Flash point: | 160-164°C/4mm |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Ethanol (Slightly, Sonicated), Methanol (Slightly) |
| form | Very Dark Orange |
| pka | 14.90±0.10(Predicted) |
| color | Light yellow to yellow |
| Merck | 14,5609 |
| BRN | 80447 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 486-70-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 2H-quinolizine-1-methanol, octahydro-, (1r-trans)-(486-70-4) |
Safety information for (-)-LUPININE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P321:Specific treatment (see … on this label). P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for (-)-LUPININE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran




![SULFURIC ACID MONO-[(1R,9AR)-1-(OCTAHYDRO-QUINOLIZIN-1-YL)METHYL] ESTER](https://img.chemicalbook.in/StructureFile/ChemBookStructure21/GIF/CB8798820.gif)

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8