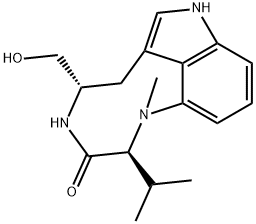
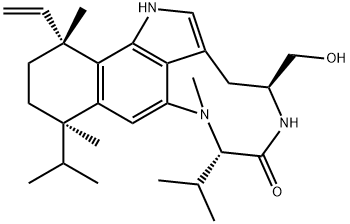
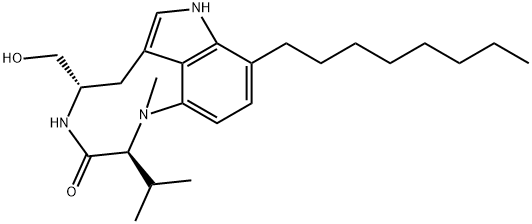
(-)-INDOLACTAM V
Synonym(s):(2S,5S)-1,2,4,5,6,8-Hexahydro-5-(hydroxymethyl)-1-methyl-2-(1-methylethyl)-3H-pyrrolo[4,3,2-gh]-1,4-benzodiazonin-3-one
- CAS NO.:90365-57-4
- Empirical Formula: C17H23N3O2
- Molecular Weight: 301.38
- MDL number: MFCD00151197
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is (-)-INDOLACTAM V?
Description
Indolactam V (-) (90365-57-4) is a PKC activator acting at the phorbol ester binding site.1,2?Directs differentiation of human and mouse ESCs to pancreatic cells.3?? May be used, along with other agents, to differentiate human iPS cells into glucose-responsive insulin-secreting progeny.4?Increases regulator of G protein signaling 2 (RGS2) protein levels, a protein that regulates GPCR signaling.5
The Uses of (-)-INDOLACTAM V
(-)-Indolactam V is an indole alkaloid compound which activates protein kinase C (PKC). Weak tumor promoter. Also directs differentiation of human embryonic stem cells, potentially into pancreatic lineage allowing for an effective diabetes therapy.
What are the applications of Application
(?)-Indolactam V is a protein kinase C activator; also can direct the differentiation of human ESCs
Definition
ChEBI: (-)-indolactam V is a 9-membered ring that is a potent protein kinase C activator
Biological Activity
Protein kinase C activator. Exhibits tumor promoting activity. Directs differentiation of human embryonic stem cells (ESCs) into pancreatic progenitors.
Biochem/physiol Actions
(-)-Indolactam V is a PKC activator shown to effect differentiation in embryonic stem cells leading to development of pancreatic precursors. It is active in the mouse model.
storage
-20°C (desiccate)
References
1) Fujiki?et al.?(1984),?Structure-activity studies on synthetic analogues (indolactams) of the tumor promoter teleocidin; Gan., 75?866 2) Heikkila and Ackerman (1989),?(-)-Indolactam V activates protein kinase C and induces changes in muscarinic receptor functions in SH-SY5Y human neuroblastoma cells; Biochem. Biophys. Res. Commun,?162?1207 3) Chen?et al.?(2009),?A small molecule that directs differentiation of human ESCs into the pancreatic lineage; Nat. Chem. Biol.,?5?258 4) Thalava?et al. (2011),?Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secretin progeny; Gene Ther.,?18?283 5) Raveh (2014),?Identification of protein kinase C activation as a novel mechanism for RGS2 protein upregulation through phenotypic screening of natural product extracts; Mol. Pharmacol.,?86?406
Properties of (-)-INDOLACTAM V
| Boiling point: | 584.0±50.0 °C(Predicted) |
| Density | 1.166±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: >10mg/mL, clear |
| form | white powder |
| pka | 14.39±0.10(Predicted) |
| color | White to light yellow |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. |
Safety information for (-)-INDOLACTAM V
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P312:Call a POISON CENTER or doctor/physician if you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
Computed Descriptors for (-)-INDOLACTAM V
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 (−)-Indolactam V CAS 90365-57-4View Details
(−)-Indolactam V CAS 90365-57-4View Details
90365-57-4 -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
