(+)-FENFLURAMINE HYDROCHLORIDE
- CAS NO.:3239-44-9
- Empirical Formula: C12H16F3N
- Molecular Weight: 231.26
- MDL number: MFCD00069276
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
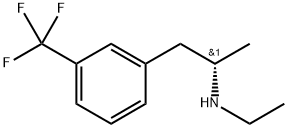
What is (+)-FENFLURAMINE HYDROCHLORIDE?
Absorption
Well-absorbed from the gastrointestinal tract.
Toxicity
Symptoms of overdose include respiratory failure and cardiac arrest leading to death.
Originator
Dexfenfluramine,Interneuron Pharmaceuticals (Lexington, Mass), for Wyeth Laboratories Inc
The Uses of (+)-FENFLURAMINE HYDROCHLORIDE
Appetite suppressant (systemic).
Indications
For the management of obesity including weight loss and maintenance of weight loss in patients on a reduced calorie diet
Background
Dexfenfluramine, also marketed under the name Redux, is a serotoninergic anorectic drug. For a fairly limited time during the middle of the nineties, the US FDA had approved it for use in managing weight loss. However, following multiple concerns about the cardiovascular side-effects of the drug, such approval was withdrawn.
Definition
ChEBI: The S-enantiomer of fenfluramine. It stimulates the release of serotonin and selectively inhibits its reuptake, but unlike fenfluramine it does not possess catecholamine agonist activity. It was formerly given by mouth as the hydrochloride in the treatment of obesity, but, like fenfluramine, was withdrawn wolrdwide following reports of valvular heart defects.
Manufacturing Process
To 10.65 parts acetic anhydride there were added, with cooling, 8 parts 1-(3-
trifluoromethylphenyl)-2-aminopropane and 100 parts water. The mixture was
neutralized with 30 parts sodium carbonate. The organic layer was extracted
twice with 50 parts ether. The ether solutions were washed with 25 parts
water and dried over potassium carbonate. On distillation there were obtained
9 parts 1-(3-trifluoromethylphenyl)-2-acetyl-aminopropane. 9 parts of it were
reduced in solution in 100 parts ether with 1.7 parts lithium and aluminium
hydride with 20 parts ether. The suspension was refluxed for 4 hours,
hydrolysed with 2 parts water, 2 parts 4 N sodium hydroxide and then 6 parts
water. The precipitate was drained washed with 50 parts ether, the filtrate was
extracted twice with 50 parts 0.5 N sulfuric acid. The acidic layers were
separated by sedimentation and neutralized with 100 parts 4 N sodium
hydroxide, the separated amine was extracted with 200 parts ether. There
were obtained 6 parts 1-(3-trifluoromethylphenyl)-2-ethylaminopropane
(boiling point 108°-112°C at 12 mm). The hydrochloride thereof was
recrystallized from mixture of ethyl alcohol and ether (melting point 166°C).
S-Isomer was prepared the next way. To a solution of 160 parts of dibenzoyl
d-tartaric acid in 1600 parts of anhydrous ethanol were added for 15 minutes
80 parts of dl-1-(3-trifluoromethylphenyl)-2-ethylaminopropane. After 15
additional minutes, 90.5 parts of crystalline solid were isolated. When this
product was recrystallized from 1300 parts of anhydrous ethanol, there was
obtained 70 parts of dibenzoyl d-tartarate acid salt of L-1-(3-
trifluoromethylphenyl)-2-ethylaminopropane. This salt was treated with 500
parts of 4 N NaOH. The mixture was extracted with 2x200-part portions of
diethyl ether and the ether extract was re-extracted with 100 parts of 4 N
hydrodiboric acid. After treatment with 120 parts of 4 N NaOH, the free amine
amounting to 25 parts distills at 105°-107°C (17.5 mm.). [α]D25: - 9.6°
(c=8% in ethanol).
brand name
Redux (Interneuron);Diomeride.
Therapeutic Function
Antiobesity
Pharmacokinetics
Used to treat diabetes and obesity, Dexfenfluramine decreases caloric intake by increasing serotonin levels in the brain’s synapses. Dexfenfluramine acts as a serotonin reuptake inhibitor. It also causes release of serotonin from the synaptosomes.
Metabolism
Not Available
Properties of (+)-FENFLURAMINE HYDROCHLORIDE
| Boiling point: | 108-112 °C(Press: 12 Torr) |
| alpha | D25 +9.5° (c = 8 in ethanol) |
| Density | 1.078±0.06 g/cm3(Predicted) |
| pka | 10.23±0.19(Predicted) |
Safety information for (+)-FENFLURAMINE HYDROCHLORIDE
Computed Descriptors for (+)-FENFLURAMINE HYDROCHLORIDE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![(S)-2-[(Bis(3,5-bis(trifluoromethyl)phenyl)trimethylsilanyloxy)methyl]pyrrolidine, (S)-2-[(Bis(3,5-bis(trifluoromethyl)phenyl)trimethylsilyloxy)methyl]pyrrolidine, (S)-α,α-[3,5-Bis(trifluoromethyl)phenyl]prolinol trimethylsilyl ether](https://img.chemicalbook.in/CAS/GIF/848821-61-4.gif)





![(S)-2-{Bis[3,5-bis(trifluoromethyl)phenyl]methoxy-methyl}pyrrolidine hydrochloride](https://img.chemicalbook.in/CAS/20200401/GIF/CB62101664.gif)
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4