(+)-EPIBATIDINE DIHYDROCHLORIDE
- CAS NO.:148152-66-3
- Empirical Formula: C11H13ClN2
- Molecular Weight: 208.69
- MDL number: MFCD00923801
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-08 16:27:35
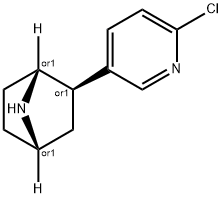
What is (+)-EPIBATIDINE DIHYDROCHLORIDE?
The Uses of (+)-EPIBATIDINE DIHYDROCHLORIDE
(-)-Epibatidine has binding affinities for α4β2 and α3β4 nicotinic receptors.
Definition
ChEBI: 3-(6-chloro-3-pyridinyl)-7-azabicyclo[2.2.1]heptane is an alkaloid.
Biological Activity
(+)-aj 76 hydrochloride is an antagonist of dopamine autoreceptor with pki values of 6.95, 6.67, 6.37, 6.21 and 6.07 for hd3, hd4, hd2s, hd2l and rd2 receptors, respectively.dopamine receptor is a g protein-coupled receptor and mainly exists in the vertebrate central nervous system (cns). dopamine receptor is a receptor for dopamine and plays a critical role in memory, learning, pleasure, cognition, motivation and fine motor control.(+)-aj 76 hydrochloride is a dopamine receptor antagonist. in rats, aj76 stimulated locomotor activity and increased the levels of 3,4-dihydroxyphenylacetic acid (dopac) and hva in brain, which were dopamine metabolites [1]. in rats injected with cocaine, (+)-a j76 increased the locomotor stimulation during the first 30 min. however, (+)-aj76 inhibited the later more intense locomotor stimulation and cocaine-induced stereotypies [2]. in vivo, (+)-aj76 induced dopamine release mainly through interaction with dopamine receptors in the terminal regions of the a9 and a10 dopaminergic fibers. however, (+)-aj76 increased the level of dopac via the somatodendritic autoreceptors [3].
storage
Desiccate at +4°C
References
[1]. kullingsjö h, carlsson a, svensson k. effects of repeated administration of the preferential dopamine autoreceptor antagonist, (+)-aj76, on locomotor activity and brain da metabolism in the rat. eur j pharmacol, 1991, 205(3): 241-246.
[2]. piercey mf, lum jt, hoffmann we, et al. antagonism of cocaine's pharmacological effects by the stimulant dopaminergic antagonists, (+)-aj76 and (+)-uh232. brain res, 1992; 588(2): 217-222.
[3]. waters n, hansson l, löfberg l, et al. intracerebral infusion of (+)-aj76 and (+)-uh232: effects on dopamine release and metabolism in vivo. eur j pharmacol, 1994, 251(2-3): 181-190.
Properties of (+)-EPIBATIDINE DIHYDROCHLORIDE
| Boiling point: | 336.7±32.0 °C(Predicted) |
| Density | 1.223±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | insoluble in H2O; <24.55mg/ml in ethanol |
| form | solid |
| pka | 10.07±0.40(Predicted) |
| color | Off White |
| Water Solubility | Soluble to 5 mM in water and to 100 mM in ethanol |
Safety information for (+)-EPIBATIDINE DIHYDROCHLORIDE
Computed Descriptors for (+)-EPIBATIDINE DIHYDROCHLORIDE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

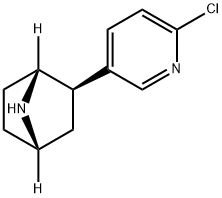
![7-Azabicyclo[2,2,1]heptane hydrochloride](https://img.chemicalbook.in/CAS/GIF/27514-07-4.gif)
![3-[2-(METHYLAMINO)ETHYL]PYRIDINE DIHYDROCHLORIDE](https://img.chemicalbook.in/StructureFile/ChemBookStructure7/GIF/CB8704294.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8