Supplier Type
- Manufacturer
- Trader
Supplier Region
- Gujarat(5)
- Kerela(1)
- Karnataka(1)
- Hyderabad(6)
- Telangana(2)
- Bangalore(1)
- New Delhi(1)
- Maharashtra(3)
- Mumbai(3)
- Rajasthan(1)
Purity
- IP/USP/BP/EP
- IP/BP-EP/USP
- IP/BP/EP/USP
- IP/BP/EP
- BP/EP/USP
- above 90 %
- 99%+
- 99% Min
- 99% and above
- 99%
- 98+
- 98.00 HPLC
- 98%+
- 98% to 99%
- 98% (HPLC)
- >98%
- 98%
- 90 % Above
Package
- 0As per requirement
- 0Metric Ton
- 25mg
- 100mg
- 250mg
- 1g
- 1gm
- 5g
- 5gm
- 10g
- 10gm
- 25gm
- 25g
- 100g
- 100gm
- 500g
- 25kg
- 256525kg in 65litre HDPE drum
- 1kg
- 5kg
- 10kg
- 25125kg and 1 MT
- 50kg
- 1 kg
- 50010500 mg and 1.0 gm
- 1MT
- 200KGS HDPE DRUM
- 2.55022e+01025/50/220 KGS HDPE DRUM / 1060 KGS IBC TANKS
- 111kg or above 1kg needs enquiry
- 255025 to 50 kg
- 100kg
- 10 kg
- 255025kg in 50litre HDPE drum
- 200Kg
- 1000Kg
- 5 kg
- 25kgs fiber /hdpe drums
- 25Kg bag
- 200Litre
- 1000Litre
- 306530kg in 65 litre HDPE drum
- 160Kg drum
-
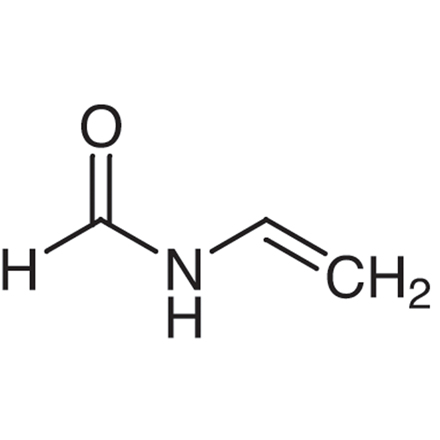
Miconazole IMpurity H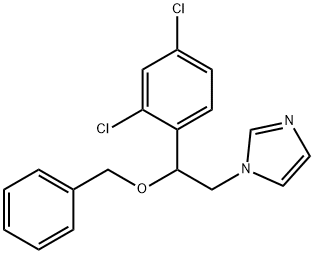
181931-30-6
-
Diacetic Aceclofenac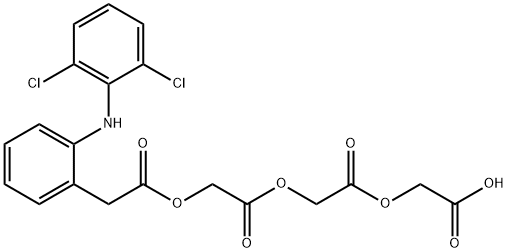
1216495-92-9
-
DoMperidone EP IMpurity D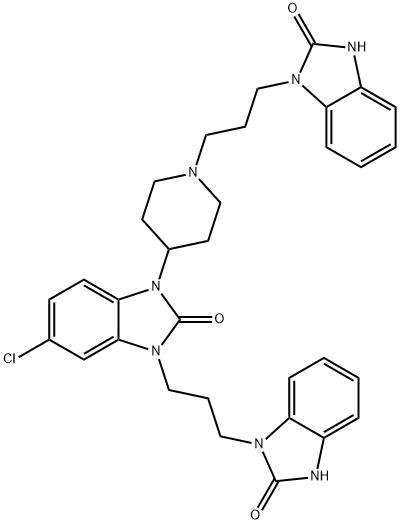
1614255-34-3
-
Aceclofenac Ethyl Ester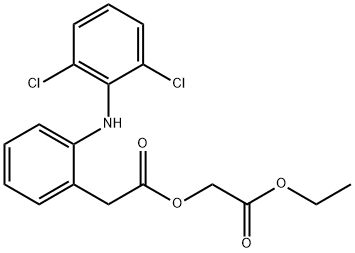
139272-67-6
-
3-Methyl-2-(3,4-dimethoxyphenyl)butyronitrile
20850-49-1
-
α,α-Bis[2-(dimethylamino)ethyl]-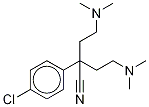
1246816-57-8
-
Flucloxacillin Sodium Impurity A
68728-50-7
-
Econazole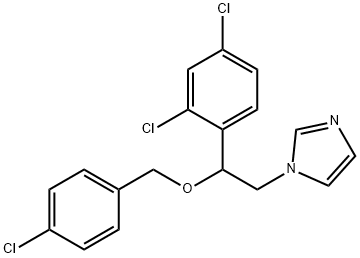
27220-47-9
-
Trityl losartan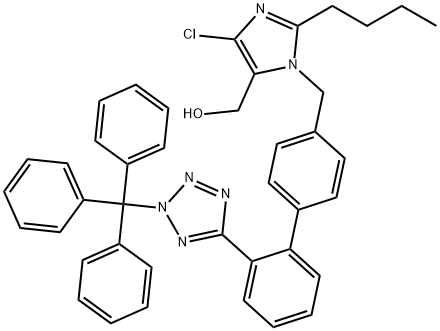
133909-99-6
-
Ethyl 4-Hydroxy-2H-1,2-benzothiazine-3-carboxylate 1,1-Dioxide (Piroxicam Impurity H)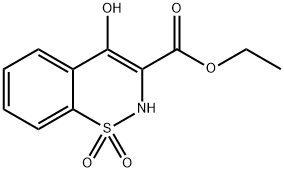
24683-21-4
1Y
Maharashtra
Phone: +91-9323789882
Tel: +91-9323789882
Whatsapp: +91- 9323789882
Web: www.nivonindia.com
Email: navonepharma@yahoo.com
Product catalog:
174
Manufacturer
Note:Purity: 99% and above | Package: 25kg in 50litre HDPE drum
2Y
Maharashtra
Phone: +919967535371
Whatsapp: +91- 9967535371
Web: www.vandvpharma.com
Email: mktg@vandvpharma.com
Product catalog:
38
2Y
Telangana
Phone: +91-9369088888
Tel: +91-9369088888
Whatsapp: +91- 9369088888
Web: aptrasynthesis.com
Email: info@aptralifesciences.com
Product catalog:
17
2Y
Rajasthan
Phone: +91-9829069545
Tel: +91-9829069545
Whatsapp: +91- 9829069545
Email: support@neelamaqua.com
Product catalog:
39
1Y
Hyderabad
Phone: +91-9398230795
Tel: +91-9398230795
Whatsapp: +91- 9398230795
Email: sales@vruddisripharma.com
Product catalog:
72
Manufacturer
500kg(MOQ)
Note:Purity: 99% | Package: 05kg;1INR|1kg;1INR|1000kg;1INR
1Y
Maharashtra
Phone: +918976372608
Whatsapp: 8976372608
Web: odechem.com
Email: sales@odechem.com
Product catalog:
27