Supplier Type
- contract manufacturer
- Manufacturer
- Trader
- custom synthesis
- Reagent
Supplier Region
- Hyderabad(18)
- Ahmedabad(5)
- Gujarat(102)
- Tamil Nadu(9)
- Telangana(47)
- Kerela(2)
- Maharashtra(99)
- Karnataka(13)
- Mumbai(14)
- Haryana(9)
- New Delhi(11)
- AndhraPradesh(4)
- Madhya Pradesh(5)
- Rajasthan(9)
- Uttar Pradesh(3)
- Vadodara(1)
- Himachal Pradesh(1)
- Andhra Pradesh(1)
Purity
- NLT-98%
- IP/USP/BP/EP
- IP/BP-EP/USP
- IP/BP/EP/USP
- IP/BP/EP
- BP/EP/USP
- above 90 %
- 99%
- 98%+
- >98%
- 98%
- 97%
- 96%
- 95%
- 90%
- 90 % Above
- 75%
- 0.98
Package
- 0Customized pack size availaible
- 1g
- 5g
- 2.55022e+01025/50/220 KGS HDPE DRUM / 1060 KGS IBC TANKS
- 0As per requirement
- 0kg
- 10MG
- 25mg
- 50mg
- 100mg
- 250mg
- 500mg
- 1gm
- 5gm
- 10gm
- 10g
- 25g
- 25gm
- 50g
- 100g
- 5kg
- 255025 to 50 kg
- 100gm
- 500g
- 1kg
- 10kg
- 25kg
- 1MT
- 10mt
- 10Kg Fiber Drum
- 25Kg HDPE Drums
- 25Kg HDPE Drum
- 25Kg Fiber Drums
- 50Ltr HDPE Drum
- 50Ltr HDPE Drums
- 1001100mg and 1 g
- 50010500 mg and 1.0 gm
- 111kg or above 1kg needs enquiry
- 20KG
- 1 kg
- 5 kg
- 10 kg
- 25125kg and 1 MT
- 50kg
- 100kg
- 1000kg
- 50Kg HDPE Drum
- 200KGS HDPE DRUM
-
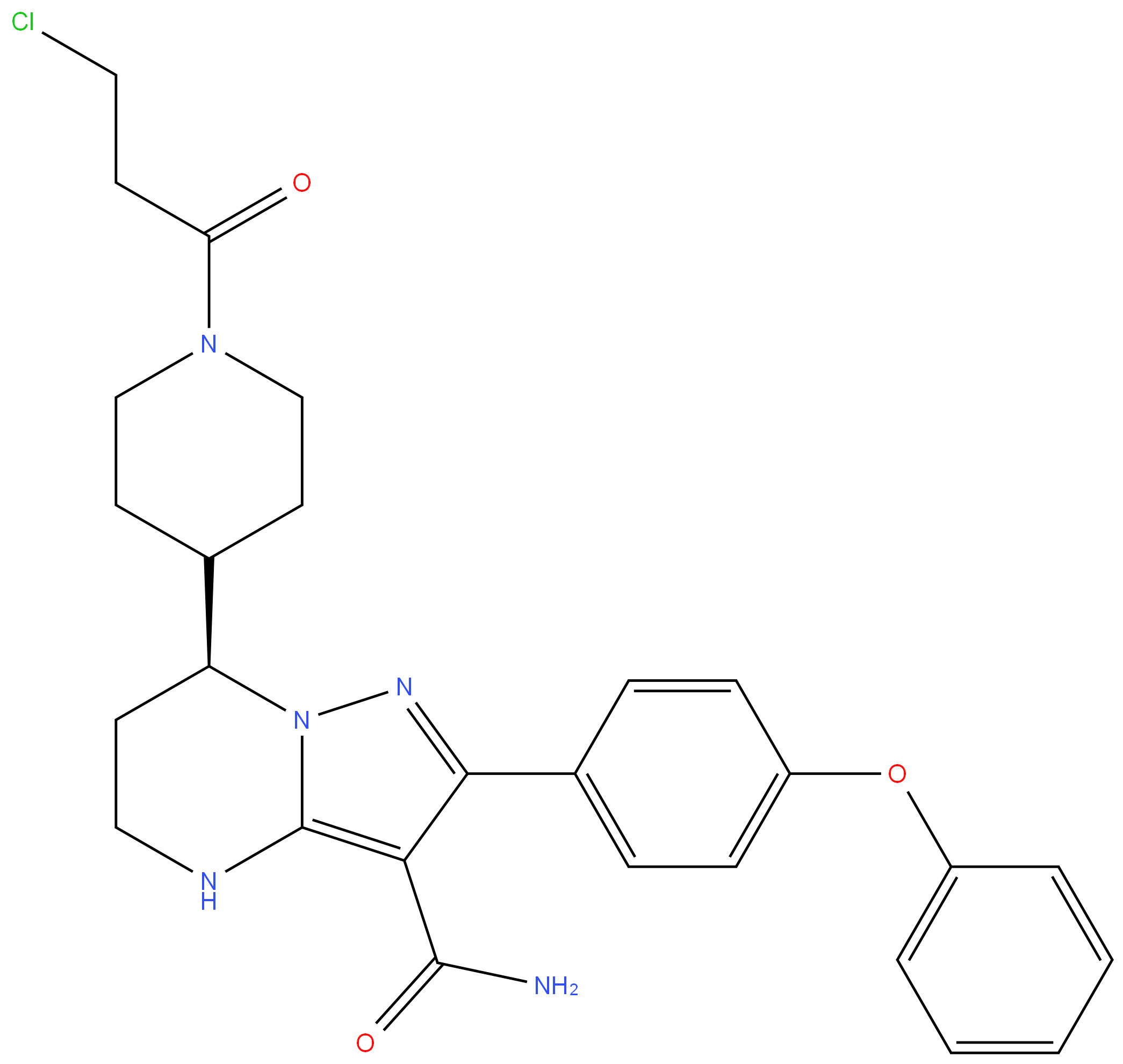
O-[(Guanin-9-yl)Methyl] Acyclovir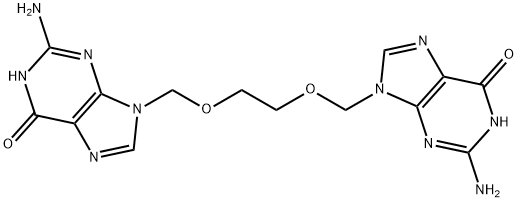
166762-90-9
-
Betamethasone EP Impurity J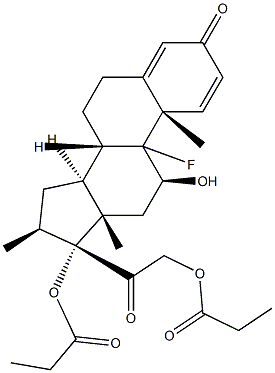
18383-24-9
-
1-Oxo Ibuprofen
65813-55-0
-
9α-BroMobudesonide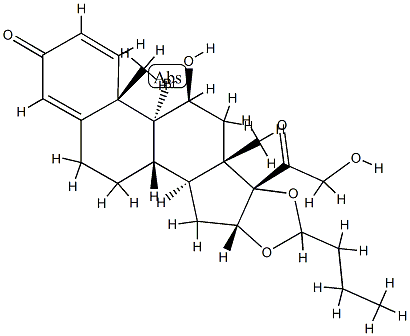
313474-59-8
-
Robenacoxib Impurity J
-
4-(3-Amino-2-hydroxypropoxy)phenylacetamide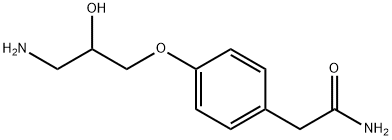
81346-71-6
-
Acebutolol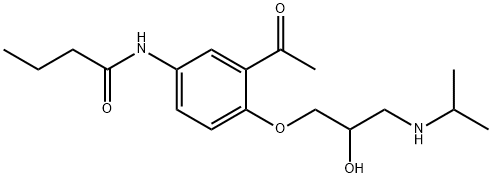
37517-30-9
-
Linagliptin iMpurity G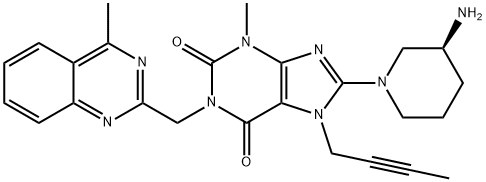
668270-11-9
-
2-(DiethylaMino)-N-(2,5-diMethylphenyl)acetaMide Hydrochloride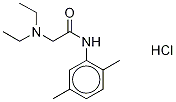
1012864-23-1
-
Acebutolol hydrochloride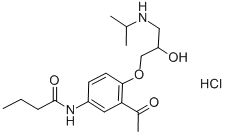
34381-68-5
Methocarbamol Dioxolone Impurity 2049-21-0
Chlorhexidine Hydrochloride 3697-42-5
Fexofenadine Hydrochloride IP/BP/USP/EP 153439-40-8
Valsartan n-Propyl Impurity 952652-79-8

Magnesium StearateI[/BP/USP/EP
Benzyltributylammonium bromide 25316-59-0
(4Z,7Z,10Z,13Z,16Z,19Z)-Docosahexaenoic acid 6217-54-5