7689-03-4
Product Name:
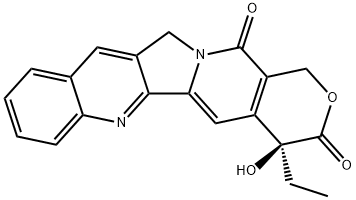
(+)-Camptothecin
Formula:
C20H16N2O4
Synonyms:
(S)-4-Ethyl-4-hydroxy-1H-pyrano-[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Melting Point | 275-277 °C |
|---|---|
| LogP | 1.74 |
| Collision Cross Section | 181.9 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H340:Germ cell mutagenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 348.4 g/mol |
|---|---|
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 348.11100700 g/mol |
| Monoisotopic Mass | 348.11100700 g/mol |
| Topological Polar Surface Area | 79.7 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 742 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Camptothecin is a pyranoindolizinoquinoline that is pyrano[3',4':6,7]indolizino[1,2-b]quinoline which is substituted by oxo groups at positions 3 and 14, and by an ethyl group and a hydroxy group at position 4 (the S enantiomer). It has a role as an EC 5.99.1.2 (DNA topoisomerase) inhibitor, an antineoplastic agent, a genotoxin and a plant metabolite. It is a pyranoindolizinoquinoline, a tertiary alcohol, a delta-lactone and a quinoline alkaloid.
RELATED SUPPLIERS
ALS India Life Sciences Pvt. Ltd
2Y
product:(S)-Camptothecin; (S)-4-Ethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione 7689-03-4 95.00%
