96036-03-2
Product Name:
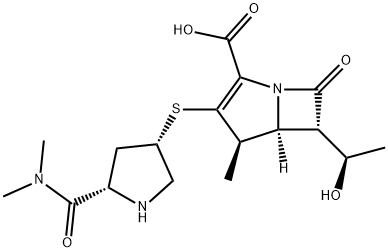
Meropenem
Formula:
C17H25N3O5S
Synonyms:
(1R,5S,6S)-2-[(3S,5S)-5-(dimethylaminocarbonyl)pyrrolidin-3-ylthio]-6-[(R)-1-hydroxyethyl]-1-methylcarbapen-2-em-3-carboxylic acid trihydrate;Meropenem trihydrate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Solubility | Sparingly |
| LogP | -0.6 |
| Stability/Shelf Life | Solutions of Meropenem (Meropenem concentrations ranging from 1 to 20 mg/mL) in Water for Injection or Sodium Chloride Injection 0.9% (for up to 4 hours) or in Dextrose Injection 5% (for up to 2 hours) at controlled room temperatures 15-25 °C (59-77 °F) are stable in plastic tubing and volume control devices of common intravenous infusion sets. Solutions of Meropenem (Meropenem concentrations ranging from 1 to 20 mg/mL) in Water for Injection or Sodium Chloride Injection 0.9% (for up to 48 hours) or in Dextrose Injection 5% (for up to 6 hours) are stable at 4 °C (39 °F) in plastic syringes. |
| Collision Cross Section | 192.42 Ų [M-H]- 190.79 Ų [M+H]+ |
| Other Experimental Properties | White to pale yellow crystalline powder. Sparingly soluble in water; very slightly soluble in hydrated ethanol /Meropenem trihydrate/ |
COMPUTED DESCRIPTORS
| Molecular Weight | 383.5 g/mol |
|---|---|
| XLogP3 | -2.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 383.15149208 g/mol |
| Monoisotopic Mass | 383.15149208 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 679 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Meropenem is a carbapenemcarboxylic acid in which the azetidine and pyrroline rings carry 1-hydroxymethyl and in which the azetidine and pyrroline rings carry 1-hydroxymethyl and 5-(dimethylcarbamoyl)pyrrolidin-3-ylthio substituents respectively. It has a role as an antibacterial drug, an antibacterial agent and a drug allergen. It is a carbapenemcarboxylic acid, a pyrrolidinecarboxamide, an alpha,beta-unsaturated monocarboxylic acid and an organic sulfide.
