95-83-0
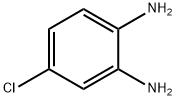
Product Name:
4-Chloro-o-phenylenediamine
Formula:
C6H7ClN2
Synonyms:
4-Chloro-1,2-diaminobenzene
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | 4-chloro-o-phenylenediamine appears as brown crystalline solid or powder. (NTP, 1992) |
|---|---|
| Color/Form | Crystals |
| Melting Point | 153 to 158 °F (NTP, 1992) |
| Solubility | less than 1 mg/mL at 66 °F (NTP, 1992) |
| Vapor Pressure | 0.00206 [mmHg] |
| LogP | log Kow = 1.28 |
| Chemical Classes | Nitrogen Compounds -> Amines, Aromatic |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 142.58 g/mol |
|---|---|
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 142.0297759 g/mol |
| Monoisotopic Mass | 142.0297759 g/mol |
| Topological Polar Surface Area | 52 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 97.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
4-chloro-o-phenylenediamine appears as brown crystalline solid or powder, it can be used as an oxidation base for dye preparation, as a chemical intermediate to produce 5-chloro benzotriazole, as a curing agent for epoxy resins, as a reagent in gas chromatography, and to synthesize experimental pharmaceuticals.
