79-11-8
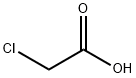
Product Name:
Chloroacetic acid
Formula:
C2H3ClO2
Synonyms:
Chloroacetic acid;Monochloroacetic acid
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Chloroacetic acid, solid is a colorless to light-brown crystalline material. It is soluble in water and sinks in water. Combustible. It is transported as a molten liquid and therefore can cause thermal burns. It is toxic by ingestion, skin absorption and inhalation of dust. It is corrosive to metals and tissue. |
|---|---|
| Color/Form | Monoclinic prisms |
| Odor | Strong vinegar-like odor |
| Boiling Point | 372 °F at 760 mmHg (EPA, 1998) |
| Melting Point | 145 °F (EPA, 1998) |
| Flash Point | 302 °F (EPA, 1998) |
| Solubility | greater than or equal to 100 mg/mL at 68 °F (NTP, 1992) |
| Density | 1.4043 at 104 °F (EPA, 1998) - Denser than water; will sink |
| Vapor Density | 3.26 (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 1 mmHg at 109.4 °F (EPA, 1998) |
| LogP | log Kow = 0.22 |
| Henry's Law Constant | Henry's Law constant = 9.26X10-9 atm-cu m/mole at 25 °C |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Autoignition Temperature | >932 °F (>500 °C) |
| Decomposition | Hazardous decomposition products formed under fire conditions: Carbon oxides, hydrogen chloride gas. |
| Corrosivity | ...extremely corrosive and will cause serious chemical burns. |
| Heat of Combustion | -715.9 kJ/mol (alpha-chloroacetic acid) |
| Heat of Vaporization | 250 Btu/Lb = 139 cal/g = 5.82X10+5 J/kg |
| Surface Tension | 35.17 dyn/cm at 100 °C |
| Odor Threshold | Odor Threshold Low: 0.04 [mmHg] Odor Threshold High: 6.14 [mmHg] [HSDB] Odor threshold from CHEMINFO |
| Refractive Index | Index of refraction: 1.4351 at 55 °C/D |
| Dissociation Constants | pKa = 2.87 |
| Other Experimental Properties | /Chloroacetic acid occurs in/ monoclinically prismatic structures existing in the alpha, beta, gamma, and also possibly the delta form. Of these the alpha form is the most stable and the most important industrially. |
| Chemical Classes | Other Classes -> Organic Acids |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 94.50 g/mol |
|---|---|
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 93.9821570 g/mol |
| Monoisotopic Mass | 93.9821570 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 42.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Chloroacetic acid, solid is a colorless to light-brown crystalline material. It is soluble in water and sinks in water. Combustible. It is transported as a molten liquid and therefore can cause thermal burns. It is toxic by ingestion, skin absorption and inhalation of dust. It is corrosive to metals and tissue.
