9003-32-1
Product Name:
POLY(ETHYL ACRYLATE)
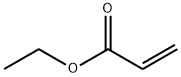
Formula:
C5H8O2
Synonyms:
Acrylic acid ethyl ester polymer;Ethyl acrylate homopolymer
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Ethyl acrylate, stabilized appears as a clear colorless liquid with an acrid odor. Flash point 60 °F. May polymerize exothermically if heated or contaminated. If the polymerization takes place inside a container, the container may rupture violently. Auto ignition temperature 721 °F (383 °C) (NTP). Less dense than water. Vapors heavier than air. Used to make paints and plastics. |
|---|---|
| Color/Form | Colorless liquid |
| Odor | Acrid penetrating odor |
| Boiling Point | 211.6 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -96.2 °F (NTP, 1992) |
| Flash Point | 48 °F (NTP, 1992) |
| Solubility | 10 to 50 mg/mL at 70 °F (NTP, 1992) |
| Density | 0.923 at 68 °F (USCG, 1999) - Less dense than water; will float |
| Vapor Density | 3.45 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 29.3 mmHg at 68 °F ; 40 mmHg at 79 °F (NTP, 1992) |
| LogP | log Kow = 1.32 |
| Stability/Shelf Life | Easily polymerizes on standing; polymerization process speeded up by heat, light, and peroxides. If pure, the monomer can be stored below +10 °C without incurring polymerization. |
| Autoignition Temperature | 721 °F (USCG, 1999) |
| Decomposition | When heated to decomp it emits acrid smoke and irritating fumes. |
| Heat of Combustion | 655.49 kcal/mol |
| Heat of Vaporization | 8.27 kcal/mol |
| Surface Tension | 0.025 DYNES/CM= 0.025 N/M @ 20 °C |
| Ionization Potential | 10.30 eV |
| Polymerization | Hazardous polymerization may occur. Polymerization may be caused by elevated temperature, oxidizers, peroxides ... Uninhibited monomer vapor may form polymer in vents and other confined spaces. /Ethyl acrylate, inhibited/ |
| Odor Threshold | Odor Threshold Low: 0.0002 [mmHg] Odor Threshold High: 0.0013 [mmHg] Detection odor threshold from AIHA (mean = 0.00024 ppm) |
| Refractive Index | Index of refraction: 1.4068 at 20 °C/D |
| Relative Evaporation Rate | 3.3 (butyl acetate= 1) |
| Kovats Retention Index | 678 683 676 678 681 680 676 684 664 664 681 681 677.6 675.1 682.8 664 677 |
| Other Experimental Properties | Polymerizes on standing; polymerization process speeded up by heat, light, and peroxides. If pure, the monomer can be stored below +10 °C without polymerization. |
| Chemical Classes | Plastics & Rubber -> (Meth)acrylates |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P281:Use personal protective equipment as required. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 100.12 g/mol |
|---|---|
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 100.052429494 g/mol |
| Monoisotopic Mass | 100.052429494 g/mol |
| Topological Polar Surface Area | 26.3 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 76.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ethyl acrylate, stabilized appears as a clear colorless liquid with an acrid odor. Flash point 60 °F. May polymerize exothermically if heated or contaminated. If the polymerization takes place inside a container, the container may rupture violently. Auto ignition temperature 721 °F (383 °C) (NTP). Less dense than water. Vapors heavier than air. Used to make paints and plastics.