14167-18-1
Product Name:
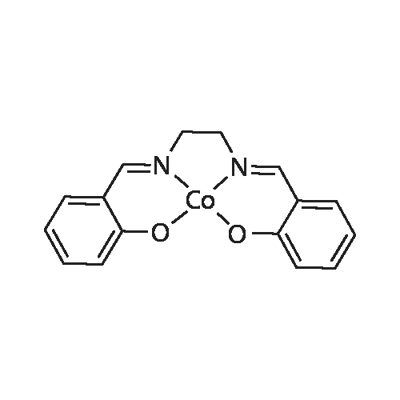
SALCOMINE
Formula:
C16H14CoN2O2
Synonyms:
N,N′-Disalicylidene-ethylenediamine cobalt(II) salt;Co(salen);Co(SALEN)2;Ethylenebis(salicylimine) cobalt(II) salt;Salcomine
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Salcomine appears as a solid complex between the cobalt(II) ion and the condensation product of salicylaldehyde and ethylenediamine. Such condensation products are called "Schiff's bases." Complexation with cobalt nullifies their basic properties. Used as a catalyst for various oxidation reactions in which it behaves as an oxygen carrier. |
|---|---|
| Color/Form | Red crystals from DMF |
| Solubility | Soluble in benzene, chloroform, and pyridine |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | When heated to decomposition it emits toxic fumes of /nitrous oxide/. |
| Other Experimental Properties | Ionization energy (IE): 7.52 eV |
| Chemical Classes | Metals -> Organic Compounds, Metal Salts |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 325.23 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 325.038721 g/mol |
| Monoisotopic Mass | 325.038721 g/mol |
| Topological Polar Surface Area | 70.8 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 298 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Salcomine appears as a solid complex between the cobalt(II) ion and the condensation product of salicylaldehyde and ethylenediamine. Such condensation products are called "Schiff's bases." Complexation with cobalt nullifies their basic properties. Used as a catalyst for various oxidation reactions in which it behaves as an oxygen carrier.
