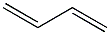| Physical Description |
Butadiene is a colorless gas with an aromatic odor. It is shipped as a liquefied gas under its vapor pressure. Contact with the liquid can cause frostbite. It is easily ignited. Its vapors are heavier than air and a flame can flash back to the source of leak very easily. It can asphyxiate by the displacement of air. It must be shipped inhibited as butadiene is liable to polymerization. If polymerization occurs in the container, it may violently rupture. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. It is used to make synthetic rubber and plastics, and to make other chemicals. |
| Color/Form |
Colorless gas ... [Note: A liquid below 24 degrees F. Shipped as a liquefied compressed gas] |
| Odor |
MIldly aromatic odor |
| Boiling Point |
24.1 °F at 760 mmHg (NTP, 1992) |
| Melting Point |
-164 °F (NTP, 1992) |
| Flash Point |
-105 °F (NTP, 1992) |
| Solubility |
Insoluble (NTP, 1992) |
| Density |
0.621 at 68 °F (USCG, 1999) - Less dense than water; will float |
| Vapor Density |
1.87 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure |
1840 mmHg at 70 °F ; 760 mmHg at 23.9 °F (NTP, 1992) |
| LogP |
log Kow = 1.99 |
| Stability/Shelf Life |
Stable under recommended storage conditions. Test for peroxide formation before using or discard after 3 months. |
| Autoignition Temperature |
788 °F (USCG, 1999) |
| Decomposition |
May decompose explosively when heated above 200 °C/ 1 kbar. ... When heated to decomposition it emits acrid smoke and fumes. |
| Viscosity |
Gas at 101.325 kPa at 20 °C: 0.00754 cP; Liquid at -40 °C: 0.33 cP |
| Corrosivity |
Non-corrosive |
| Heat of Combustion |
-2541.5 kJ/mol (gas) |
| Heat of Vaporization |
20.86 kJ/mol 25 °C; 22.47 kJ/mol at -4.41 °C |
| Surface Tension |
13.4 dynes/cm at 20 °C |
| Ionization Potential |
9.07 eV |
| Polymerization |
POLYMERIZES & COPOLYMERIZES EASILY, EG,UNDER INFLUENCE OF SODIUM ... |
| Odor Threshold |
Odor Threshold Low: 0.09 [ppm] Odor Threshold High: 76.0 [ppm] Detection odor threshold from AIHA (mean = 0.45 ppm) |
| Refractive Index |
Index of refraction: 1.4292 at 25 °C |
| Relative Evaporation Rate |
Greater than 25 (butyl acetate = 1) |
| Kovats Retention Index |
403 395 395 393 395 394.3 397 405 394 395 395 389 396 390 392 393 398 395 412 |
| Other Experimental Properties |
Dimerizes to 4-vinylcyclohexane |
| Chemical Classes |
Plastics & Rubber -> Other Monomers |