87893-55-8
Product Name:
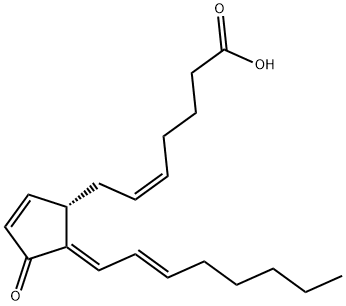
15D-PGJ2
Formula:
C20H28O3
Synonyms:
11-Oxoprosta-(5Z,9,12E,14E)-tetraen-1-oic acid;15-Deoxy-Δ12,14-PGJ2;15d-PGJ₂ - CAS 87893-55-8 - Calbiochem;Prostaglandin J₂, 15-Deoxy-Δ 12,14, PPAR Agonist VII, PPARγ Agonist VI
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| LogP | 3.983 |
| Collision Cross Section | 192.5 Ų [M+Na]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)] |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 316.4 g/mol |
|---|---|
| XLogP3 | 5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 11 |
| Exact Mass | 316.20384475 g/mol |
| Monoisotopic Mass | 316.20384475 g/mol |
| Topological Polar Surface Area | 54.4 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 495 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
15-deoxy-Delta(12,14)-prostaglandin J2 is a prostaglandin J derivative comprising prostaglandin J2 lacking the 15-hydroxy group and having C=C double bonds at the 12- and 14-positions. It has a role as a metabolite, an electrophilic reagent and an insulin-sensitizing drug. It is functionally related to a prostaglandin J2. It is a conjugate acid of a 15-deoxy-Delta(12,14)-prostaglandin J2(1-).