865854-05-3
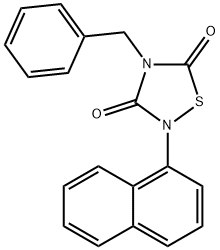
Product Name:
4-Benzyl-2-(naphthalen-1-yl)-[1,2,4]thiadiazolidine-3,5-dione
Formula:
C19H14N2O2S
Synonyms:
4-Benzyl-2-(naphthalen-1-yl)-[1,2,4]thiadiazolidine-3,5-dione;NP031112;NP-12
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Boiling Point | 511.3ºC at 760 mmHg |
|---|---|
| Melting Point | 148-150ºC |
| Solubility | Sparingly soluble |
| LogP | 3.28 |
COMPUTED DESCRIPTORS
| Molecular Weight | 334.4 g/mol |
|---|---|
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 334.07759887 g/mol |
| Monoisotopic Mass | 334.07759887 g/mol |
| Topological Polar Surface Area | 65.9 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 492 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Tideglusib is a member of the class of thiadiazolidines that is 1,2,4-thiadiazolidine-3,5-dione which is substituted by a naphthalen-1-yl group at position 2 and by a benzyl group at position 4. It is a non-ATP competitive inhibitor of glycogen synthase kinase 3beta (GSK3beta) and has neuroprotective effects. Currently under clinical investigation for the treatment of Alzheimer's disease and progressive supranuclear palsy. It has a role as an EC 2.7.11.26 (tau-protein kinase) inhibitor, a neuroprotective agent, an anti-inflammatory agent and an apoptosis inducer. It is a member of naphthalenes, a member of benzenes and a thiadiazolidine.
