391210-10-9
Product Name:
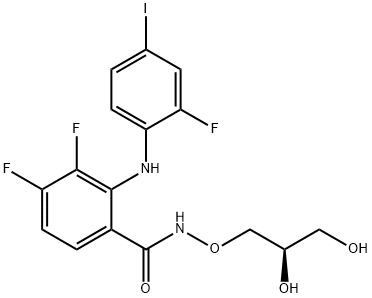
PD 0325901
Formula:
C16H14F3IN2O4
Synonyms:
InSolution MEK1/2 Inhibitor III, PD0325901 - CAS 391210-10-9 - Calbiochem;MEK1/2 Inhibitor III - CAS 391210-10-9 - Calbiochem;N-[(2R)-2,3-Dihydroxypropoxy]-3,4-difluoro-2-[(2-fluoro-4-iodophenyl)amino]-benzamide;PD 0325901, N-((2R)-2,3-Dihyroxypropoxy)-3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)-benzamide, PD325901;PD325901, PD0325901, N-((2R)-2,3-Dihyroxypropoxy)-3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)-benzamide, PD 0325901, MEK Inhibitor III
Inquiry
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H373:Specific target organ toxicity, repeated exposure H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 482.19 g/mol |
|---|---|
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 7 |
| Exact Mass | 481.99504 g/mol |
| Monoisotopic Mass | 481.99504 g/mol |
| Topological Polar Surface Area | 90.8 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 465 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
PD 0325901 is a hydroxamic acid ester that is benzhydroxamic acid (N-hydroxybenzamide) in which the hydroxamic acid group has been converted to the corresponding 2,3-dihydroxypropyl ester and in which the benzene ring has been substituted at position 2 by a (2-fluoro-4-iodophenyl)amino group and at positions 3 and 4 by fluorines (the R enantiomer). It has a role as an EC 2.7.12.2 (mitogen-activated protein kinase kinase) inhibitor and an antineoplastic agent. It is a hydroxamic acid ester, a secondary amino compound, a member of monofluorobenzenes, an organoiodine compound, a member of propane-1,2-diols and a difluorobenzene.
