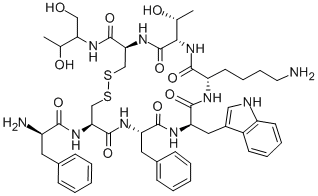
83150-76-9
CHEMICAL AND PHYSICAL PROPERTIES
| Boiling Point | 1447 |
|---|---|
| Melting Point | 153-156 |
COMPUTED DESCRIPTORS
| Molecular Weight | 1019.2 g/mol |
|---|---|
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 13 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 17 |
| Exact Mass | 1018.44048069 g/mol |
| Monoisotopic Mass | 1018.44048069 g/mol |
| Topological Polar Surface Area | 383 Ų |
| Heavy Atom Count | 71 |
| Formal Charge | 0 |
| Complexity | 1740 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Acromegaly is a disorder caused by excess growth hormone (GH), increasing the growth of body tissues and causing metabolic dysfunction. In most cases, it results from an anterior pituitary growth hormone-releasing tumor. Typically, the feet, hands, and face grow abnormally large; organomegaly and insulin resistance may also occur. Acromegaly is a life-threatening disease requiring life-long management. Octreotide is a long-acting drug with pharmacologic activities that mimic those of the natural hormone, somatostatin, which inhibits the secretion of growth hormone. Additionally, it is used for the treatment of acromegaly and symptoms arising from various tumors, including carcinoid tumors and vasoactive intestinal tumors (VIPomas). In the past, octreotide has been administered solely by injection. On June 26, 2020, the first approved delayed-release oral somatostatin analog, Mycapssa, received FDA approval for the long term maintenance treatment of acromegaly. This drug was developed by Chiasma Inc.