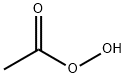
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Peracetic acid is a colorless liquid with a strong, pungent acrid odor. Used as a bactericide and fungicide, especially in food processing; as a reagent in making caprolactam and glycerol; as an oxidant for preparing epoxy compounds; as a bleaching agent; a sterilizing agent; and as a polymerization catalyst for polyester resins. (EPA, 1998) |
|---|---|
| Color/Form | Colorless liquid |
| Odor | Acrid |
| Boiling Point | 221 °F at 760 mmHg (EPA, 1998) |
| Melting Point | -22 to 32 °F (EPA, 1998) |
| Flash Point | 105 °F Peracetic Acid, 60% Acetic Acid Solution (EPA, 1998) |
| Solubility | Very soluble in ether, sulfuric acid; soluble in ethanol |
| Density | 1.226 at 59 °F (EPA, 1998) - Denser than water; will sink |
| Vapor Density | Relative vapor density (air = 1): 2.6 |
| Vapor Pressure | 14.5 [mmHg] |
| Henry's Law Constant | Henry's Law constant = 2.14X10-6 atm-cu m/mol at 25 °C |
| Stability/Shelf Life | Thermally unstable. |
| Autoignition Temperature | 392 °F (USCG, 1999) |
| Decomposition | May decompose explosively. |
| Viscosity | 3.280 cP at 78 °F |
| Corrosivity | Corrosive to most metals, including aluminum |
| Refractive Index | Index of refraction = 1.3974 at 20 °C/D |
| Dissociation Constants | pKa = 8.20 at 25 °C |
| Other Experimental Properties | BP: 25 °C at 1.6 hPa. MP: 0 °C. Stable in dilute aqueous solutions. Specific gravity: 1.0375 at 20 °C/4 °C. |
| Chemical Classes | Other Classes -> Peroxides, Organic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
COMPUTED DESCRIPTORS
| Molecular Weight | 76.05 g/mol |
|---|---|
| XLogP3 | -0.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 76.016043985 g/mol |
| Monoisotopic Mass | 76.016043985 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 40.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Peracetic acid is a colorless liquid with a strong, pungent acrid odor. Used as a bactericide and fungicide, especially in food processing; as a reagent in making caprolactam and glycerol; as an oxidant for preparing epoxy compounds; as a bleaching agent; a sterilizing agent; and as a polymerization catalyst for polyester resins. (EPA, 1998)