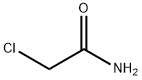
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Colorless to pale yellow crystals; [HSDB] |
|---|---|
| Color/Form | Crystals from water |
| Odor | Characteristic odor |
| Boiling Point | Approximately 225 °C (decomposes) |
| Melting Point | 119-120 °C |
| Flash Point | 170 °C |
| Solubility | In water, 90,000 mg/L at 25 °C |
| Density | Relative density of the vapour/air-mixture at 20 °C (air = 1): 1.00 |
| Vapor Density | Relative vapor density (air = 1): 3.2 |
| Vapor Pressure | 0.01 [mmHg] |
| LogP | log Kow = -0.53 |
| Stability/Shelf Life | Two isomorphous crystalline modifications: alpha-form, "stable form", obtained by sublimation and by crystallization from nonpolar solvents; beta-form, "unstable form", obtained by quenching the melt or by crystallization from polar solvents ... . |
| Decomposition | Decomposes on heating. This produces toxic fumes including nitrogen oxides and chlorine. |
| Corrosivity | Strong irritant to skin and tissue |
| Other Experimental Properties | Two isomorphous crystalline structures: alpha form "stable form", obtained by sublimation and crystallization from non polar solvents; beta form "unstable form", obtained by quenching the melt or by crystallization from polar solvents. |
| Chemical Classes | Other Uses -> Biocides/Disinfectants |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
COMPUTED DESCRIPTORS
| Molecular Weight | 93.51 g/mol |
|---|---|
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 92.9981414 g/mol |
| Monoisotopic Mass | 92.9981414 g/mol |
| Topological Polar Surface Area | 43.1 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 44.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Chloroacetamide is a chlorinated sulfhydryl alkylating reagent that forms covalent bonds with the thiol group of cysteines so the protein or peptide no longer forms disulfide bonds.