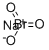CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sodium bromate appears as a white crystalline solid. May explode under prolonged exposure to heat or fire. Used in chemical analysis. |
|---|---|
| Color/Form | White crystals or white powder |
| Odor | Odorless |
| Solubility | Solubility in water (g/100 g water): 27.5 at 0 °C; 48.8 at 40 °C; 62.6 at 60 °C; 75.8 at 80 °C; 90.8 at 100 °C |
| Density | 3.200 g/cu cm |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | When heated to decomposition it emits toxic fumes of Na2O and /hydrogen bromide/. |
| pH | Aqueous solution is neutral |
| Refractive Index | Index of refraction: 1.594 |
| Other Experimental Properties | Decomposes on heating with evolution of oxygen and formation of sodium bromide |
| Chemical Classes | Other Classes -> Halogens, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H271:Oxidising liquids;Oxidising solids H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 150.89 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 149.89285 g/mol |
| Monoisotopic Mass | 149.89285 g/mol |
| Topological Polar Surface Area | 57.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 49.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium bromate appears as a white crystalline solid. May explode under prolonged exposure to heat or fire. Used in chemical analysis.