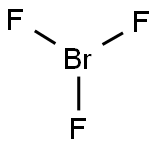CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Bromine trifluoride appears as a colorless to yellow, fuming liquid with a pungent odor. Solidifies at 48 °F. Very toxic by inhalation and corrosive to metals and tissue. Containers exposed to prolonged heat may violently rupture and rocket. |
|---|---|
| Color/Form | Colorless liquid; also reported to be pale yellow. Long prisms when solid. |
| Boiling Point | 258.4 °F at 760 mmHg (USCG, 1999) |
| Melting Point | 47.8 °F (USCG, 1999) |
| Density | 2.81 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Pressure | 2.8030 g/cu cm at 25 °C |
| Decomposition | ... If involved in a fire decomp to produce hydrogen fluoride and hydrogen bromide. |
| Other Experimental Properties | Explosive or violent reaction with organic materials |
| Chemical Classes | Toxic Gases & Vapors -> Oxidizers |
COMPUTED DESCRIPTORS
| Molecular Weight | 136.90 g/mol |
|---|---|
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 135.91355 g/mol |
| Monoisotopic Mass | 135.91355 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Bromine trifluoride appears as a colorless to yellow, fuming liquid with a pungent odor. Solidifies at 48 °F. Very toxic by inhalation and corrosive to metals and tissue. Containers exposed to prolonged heat may violently rupture and rocket.