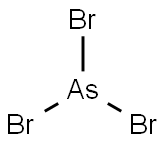
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Arsenic bromide appears as a yellowish white crystalline solid. Absorbs moisture from the air. Decomposed by water to form arsenic acid and hydrobromic acid, a corrosive. Intensely toxic. |
|---|---|
| Color/Form | Orthorhombic prisms |
| Boiling Point | 221 °C |
| Melting Point | 31.1 °C |
| Solubility | Soluble in hydrocarbons; carbon tetrachloride; very soluble in ether, benzene |
| Density | 3.40 @ 25 °C |
| Vapor Pressure | 1 mm Hg @ 41.8 °C |
| Stability/Shelf Life | FUMES IN MOIST AIR; DECOMP BY WATER FORMING ARSENIC TRIOXIDE AND HYDROGEN BROMIDE. |
| Decomposition | WHEN HEATED TO DECOMP IT EMITS VERY TOXIC FUMES OF /ARSENIC AND HYDROGEN BROMIDE/. |
| Other Experimental Properties | Deliquescent. Dipole moment: 1.66; Heat of fusion: 8.93 cal/g; |
| Chemical Classes | Metals -> Arsenic Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 314.63 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 313.67456 g/mol |
| Monoisotopic Mass | 311.67661 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Arsenic bromide appears as a yellowish white crystalline solid. Absorbs moisture from the air. Decomposed by water to form arsenic acid and hydrobromic acid, a corrosive. Intensely toxic.