CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Solubility | 7.97e+01 g/L |
| Collision Cross Section | 239.1 Ų [M+H]+ [CCS Type: TW, Method: calibrated with Waters Major Mix] |
COMPUTED DESCRIPTORS
| Molecular Weight | 615.6 g/mol |
|---|---|
| XLogP3 | -8.7 |
| Hydrogen Bond Donor Count | 13 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 9 |
| Exact Mass | 615.29630113 g/mol |
| Monoisotopic Mass | 615.29630113 g/mol |
| Topological Polar Surface Area | 347 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 870 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 19 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
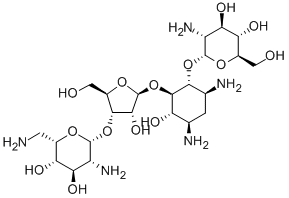
Paromomycin is an amino cyclitol glycoside that is the 1-O-(2-amino-2-deoxy-alpha-D-glucopyranoside) and the 3-O-(2,6-diamino-2,6-dideoxy-beta-L-idopyranosyl)-beta-D-ribofuranoside of 4,6-diamino-2,3-dihydroxycyclohexane (the 1R,2R,3S,4R,6S diastereoisomer). It is obtained from various Streptomyces species. A broad-spectrum antibiotic, it is used (generally as the sulfate salt) for the treatment of acute and chronic intestinal protozoal infections, but is not effective for extraintestinal protozoal infections. It is also used as a therapeutic against visceral leishmaniasis. It has a role as an antibacterial drug, an antiprotozoal drug, an anthelminthic drug and an antiparasitic agent. It is an aminoglycoside antibiotic and an amino cyclitol glycoside. It is functionally related to a streptamine.