75-52-5
Product Name:
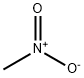
Nitromethane
Formula:
CH3NO2
Synonyms:
Mononitromethane, Nitrocarbol;Nitromethane;Nitromethane in dimethyl sulfoxide;Nitromethane solution
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Nitromethane appears as a colorless oily liquid. Flash point 95 °F. May violently decompose if intensely heated when contaminated. Denser than water and slightly soluble in water. Hence sinks in water. Vapors are heavier than air. Moderately toxic. Produces toxic oxides of nitrogen during combustion. |
|---|---|
| Color/Form | Colorless liquid |
| Odor | Moderately strong, somewhat disagreeable odor |
| Boiling Point | 214.2 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -20 °F (NTP, 1992) |
| Flash Point | 95 °F (NTP, 1992) |
| Solubility | greater than or equal to 100 mg/mL at 68 °F (NTP, 1992) |
| Density | 1.139 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | 2.11 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 27.8 mmHg at 68 °F (NTP, 1992) |
| LogP | low Kow = -0.35 |
| Henry's Law Constant | Henry's Law constant = 2.86X10-5 atm-cu m/mol at 25 °C |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Autoignition Temperature | 785 °F (USCG, 1999) |
| Decomposition | When heated to decomposition it emits toxic fumes of /nitrogen oxides/. |
| Viscosity | 0.614 cP at 25 °C |
| Heat of Combustion | -709 kJ/mol at 25 °C |
| Heat of Vaporization | 38.3 kJ/mol at 25 °C ; 34.4 kJ/mol at the boiling point |
| pH | pH of 0.01M aqueous solution = 6.12 |
| Surface Tension | 37.19 dynes/cm at 20 °C |
| Ionization Potential | 11.08 eV |
| Odor Threshold | Odor Threshold Low: 3.5 [mmHg] Odor Threshold High: 100.0 [mmHg] Odor threshold from CHEMINFO |
| Refractive Index | Index of refraction = 1.3817 at 20 °C/D |
| Dissociation Constants | pKa = 10.21 |
| Relative Evaporation Rate | 1.3 (Butyl acetate = 1); 9 (Diethyl ether = 1) |
| Kovats Retention Index | 526.13 527.75 527.85 527.88 528.15 528.16 528.6 528.66 529.26 530.05 531.15 556 543.6 536 565 565 565 531 487 526 531 521 526 565 521 536 565 |
| Other Experimental Properties | Heat of formation (liq) at 25 °C= -113.1 kJ/mol |
| Chemical Classes | Nitrogen Compounds -> Nitros, Aliphatic |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 61.040 g/mol |
|---|---|
| XLogP3 | 0.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 61.016378338 g/mol |
| Monoisotopic Mass | 61.016378338 g/mol |
| Topological Polar Surface Area | 45.8 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 27.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Nitromethane appears as a colorless oily liquid. Flash point 95 °F. May violently decompose if intensely heated when contaminated. Denser than water and slightly soluble in water. Hence sinks in water. Vapors are heavier than air. Moderately toxic. Produces toxic oxides of nitrogen during combustion.
