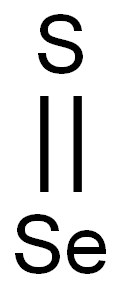CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Selenium sulfide appears as orange-yellow tablets or powder. Has a faint odor. (NTP, 1992) |
|---|---|
| Color/Form | Orange-yellow tablets or powder |
| Odor | Odorless |
| Boiling Point | Decomposes at 244-246 °F (NTP, 1992) |
| Melting Point | 231.9 °F (NTP, 1992) |
| Solubility | less than 1 mg/mL at 70 °F (NTP, 1992) |
| Density | 3.056 at 32 °F (NTP, 1992) - Denser than water; will sink |
| Decomposition | When heated to decomposition it emits very toxic fumes of /sulfur oxides and selenium/. |
| Other Experimental Properties | Paper coated with selenium sulfide blackens in mercury vapor because of formation of hydrogen sulfide |
| Chemical Classes | Other Classes -> Other Inorganic Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 111.04 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 111.88859 g/mol |
| Monoisotopic Mass | 111.88859 g/mol |
| Topological Polar Surface Area | 32.1 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Selenium sulfide appears as orange-yellow tablets or powder. Has a faint odor. (NTP, 1992)