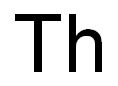CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Gray-white lustrous metal; [Merck Index] |
|---|---|
| Color/Form | Soft gray-white metal; cubic |
| Boiling Point | 4788 °C |
| Melting Point | 1750 °C |
| Solubility | Insoluble in water; soluble in hydrochloric acid, sulfuric acid, aqua regia; slightly soluble in nitric acid |
| Density | 11.7 g/cu cm |
| Vapor Pressure | Vapor pressure: log(p/atm) = -28,780 (T/K)-1 + 5991 at 1757-1956 K |
| Stability/Shelf Life | When pure it is air-stable. |
| Autoignition Temperature | 270 °C |
| Decomposition | Thorium disintegrates with production of thoron ((220)radon), which is alpha emitter and presents radiation hazard. Good ventilation of areas where thorium is stored or handled is therefore essential. |
| Heat of Vaporization | approx. 586 kJ/mol |
| Other Experimental Properties | Valance 4; no stable nuclides; heat capacity: 27.32 J/mol K at 25 °C; darkens on prolonged exposure to air; finely divided metal is pyrophoric in air; HCl attacks metal vigorously, leaving up to 25% as an undissolved residue; nitric acid passivates (protects by forming an oxide layer) metal; dilute hydrofluoric acid and sulfuric acid, and concentrated phosphoric acid and perchloric acid attack thorium slowly, with evolution of hydrogen; metal is not attacked by alkali hydroxides |
| Chemical Classes | Physical/Radiation -> Radionuclides |
COMPUTED DESCRIPTORS
| Molecular Weight | 232.038 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 232.03805 g/mol |
| Monoisotopic Mass | 232.03805 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 1 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Thorium is a naturally occurring, radioactive substance. In the environment, thorium exists in combination with other minerals, such as silica. Small amounts of thorium are present in all rocks, soil, water, plants, and animals. Soil contains an average of about 6 parts of thorium per million parts of soil (6 ppm). More than 99% of natural thorium exists in the form of thorium-232. It breaks down into two parts-a small part called "alpha" radiation and a large part called the decay product. The decay product is also not stable and continues to break down through a series of decay products until a stable product is formed. During these decay processes, radioactive substances are produced. These include radium and radon. These substances give off radiation, including alpha and beta particles, and gamma radiation. Some rocks in underground mines contain thorium in a more concentrated form. After these rocks are mined, thorium is usually concentrated and changed into thorium dioxide or other chemical forms. After most of the thorium is removed, the rocks are called "depleted" ore or tailings. Thorium is used to make ceramics, gas lantern mantles, and metals used in the aerospace industry and in nuclear reactions. Thorium can also be used as a fuel for generating nuclear energy.