74103-07-4
CHEMICAL AND PHYSICAL PROPERTIES
| Solubility | >56.5 [ug/mL] (The mean of the results at pH 7.4) |
|---|---|
| Collision Cross Section | 155.6 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 376.4 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Exact Mass | 376.16343649 g/mol |
| Monoisotopic Mass | 376.16343649 g/mol |
| Topological Polar Surface Area | 146 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 430 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
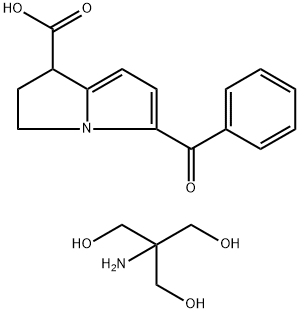
description
Ketorolac Tromethamine is the tromethamine salt of ketorolac, a synthetic pyrrolizine carboxylic acid derivative with anti-inflammatory, analgesic and antipyretic properties. Ketorolac tromethamine, a non-selective inhibitor of the cyclooxygenases (COX), inhibits both COX-1 and COX-2 enzymes. This agent exerts its anti-inflammatory effect by preventing conversion of arachidonic acid to prostaglandins at inflammation site mediated through inhibition of COX-2, which is undetectable in most tissues but is up-regulated at the inflammation sites. Since COX-1 is expressed virtually in all tissues, inhibition of COX-1 enzyme by this agent prevents normal state production of prostaglandins, which plays housekeeping roles in the protection of the gastrointestinal tract, regulating renal blood flow, and functioning in platelet aggregation. As a result, inhibition of COX-1 is usually associated with adverse effects such as gastrointestinal toxicity and nephrotoxicity.
