69256-17-3
Product Name:
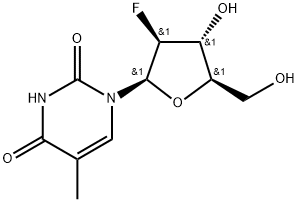
1-[(2R,3S,4R,5R)-3-Fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione
Formula:
C10H13FN2O5
Inquiry
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 260.22 g/mol |
|---|---|
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 260.08084968 g/mol |
| Monoisotopic Mass | 260.08084968 g/mol |
| Topological Polar Surface Area | 99.1 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 413 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Clevudine is a synthetic pyrimidine analogue with activity against hepatitis B virus (HBV). Intracellularly, clevudine is phosphorylated to its active metabolites, clevudine monophosphate and triphosphate. The triphosphate metabolite competes with thymidine for incorporation into viral DNA, thereby causing DNA chain termination and inhibiting the function of HBV DNA polymerase (reverse transcriptase). Clevudine has a long half-life and shows significant reduction of covalently closed circular DNA (cccDNA), therefore the patient is less likely to have a relapse after treatment is discontinued.