687-47-8
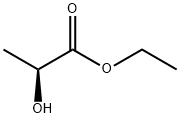
Product Name:
Ethyl L(-)-lactate
Formula:
C5H10O3
Synonyms:
(−)-Ethyl (S)-2-hydroxypropionate;(S)-(−)-2-Hydroxypropionic acid ethyl ester;(S)-(-)-Ethyl lactate;L -Lactic acid ethyl ester;L(-)-Lactic acid ethyl ester, (S)-(-)-2-Hydroxypropionic acid ethyl ester
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Liquid |
|---|---|
| Vapor Pressure | 1.6 [mmHg] |
| Kovats Retention Index | 1356 1342.3 |
| Chemical Classes | Solvents -> Esters (<C12) |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 118.13 g/mol |
|---|---|
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 118.062994177 g/mol |
| Monoisotopic Mass | 118.062994177 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 79.7 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ethyl (2S)-lactate is the (2S)-enantiomer of ethyl lactate. It has a role as a Saccharomyces cerevisiae metabolite. It is an enantiomer of an ethyl (2R)-lactate.
