67-45-8
Product Name:
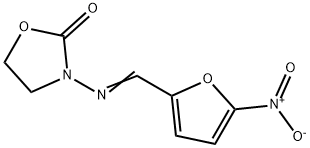
Furazolidone
Formula:
C8H7N3O5
Synonyms:
3-(5-Nitrofurfurylideneamino)-2-oxazolidinone;Furazolidone
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Yellow odorless solid; [Merck Index] |
|---|---|
| Color/Form | Yellow crystals from DMF (N,N-dimethylformamide) |
| Odor | Odorless |
| Melting Point | 254-256 |
| Solubility | 40 mg/L (at 25 °C) |
| Vapor Pressure | 0.0000026 [mmHg] |
| LogP | -0.04 |
| Decomposition | When heated to decomposition it emits toxic fumes of /nitrogen oxides/. |
| Collision Cross Section | 146.88 Ų [M+H]+ 146 Ų [M+Na]+ |
| Other Experimental Properties | Decomposed by alkali |
| Chemical Classes | Other Uses -> Animal Feed Additives |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H361:Reproductive toxicity |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 225.16 g/mol |
|---|---|
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 225.03857033 g/mol |
| Monoisotopic Mass | 225.03857033 g/mol |
| Topological Polar Surface Area | 101 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 326 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Furazolidone is a member of the class of oxazolidines that is 1,3-oxazolidin-2-one in which the hydrogen attached to the nitrogen is replaced by an N-{[(5-nitro-2-furyl)methylene]amino} group. It has antibacterial and antiprotozoal properties, and is used in the treatment of giardiasis and cholera. It has a role as an EC 1.4.3.4 (monoamine oxidase) inhibitor, an antitrichomonal drug, an antiinfective agent and an antibacterial drug. It is a member of oxazolidines and a nitrofuran antibiotic.
RELATED SUPPLIERS
Moteraa Biotech
Pune
product:Powder As Recommended By Veterinarian Furazolidone, For Poultry And Aqua, Treatment: Diseases
