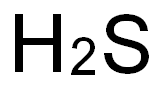| Physical Description |
Hydrogen sulfide appears as a colorless gas having a strong odor of rotten eggs. Boiling point -60.2 °C. Shipped as a liquid confined under its own vapor pressure. Density (liquid) 8.3 lb / gal. Contact with the unconfined liquid can cause frostbite by evaporative cooling. Gas is very toxic by inhalation. Fatigues the sense of smell which cannot be counted on to warn of the continued presence of the gas. Prolonged exposure of closed containers to heat may result in their violent rupturing and rocketing. Rate of onset: Immediate & Delayed Persistence: Minutes to hours Odor threshold: 0.1 ppm Source/use/other hazard: Disinfectant lubricant/oils; interm for HC manufacture; deadens sense of smell. |
| Color/Form |
Colorless gas [Shipped as a liquefied compressed gas]. |
| Odor |
Strong odor of rotten eggs [Note: Sense of smell becomes rapidly fatigued & can NOT be relied upon to warn of the continuous presense of hydrogen sulfide]. |
| Taste |
Sweetish taste |
| Boiling Point |
-76.59 °F at 760 mmHg (EPA, 1998) |
| Melting Point |
-121.9 °F (EPA, 1998) |
| Flash Point |
500 °F |
| Solubility |
0.4 % (NIOSH, 2023) |
| Density |
0.916 at -76 °F (EPA, 1998) - Less dense than water; will float |
| Vapor Density |
1.19 (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure |
15200 mmHg at 77.9 °F (EPA, 1998) |
| Henry's Law Constant |
Henry's law constant = 0.0098 atm-cu m/mole at 25 °C |
| Stability/Shelf Life |
Water solns of H2S are not stable, absorbed oxygen causes the formation of elemental sulfur, and the solns become turbid rapidly. |
| Autoignition Temperature |
500 °F (USCG, 1999) |
| Decomposition |
When heated to decomposition it emits highly toxic fumes of /oxides of sulfur/. |
| Viscosity |
Gas at 101.325 KPa at 25 °C; 0.012 8 m Pa.S; 0.012 8 cP. |
| Corrosivity |
Anhydrous hydrogen sulfide has low general corrosivity toward carbon steel, aluminum, Inconel, Stellite and 300-series stainless steels at moderate temperatures. Temperatures greater than ca 260 °C can produce severe sulfidation of carbon steel. Wet hydrogen sulfide can be quite corrosive to carbon steel. |
| Heat of Vaporization |
Molar enthalpy of vaporization: 14.08 kJ/mol at 25 °C; 18.67 kJ/mol at -59.55 °C |
| pH |
pH freshly prepared water soln: 4.5 |
| Ionization Potential |
10.46eV |
| Odor Threshold |
Odor Threshold Low: 0.001 [ppm] Odor Threshold High: 0.13 [ppm] Detection odor threshold from AIHA (mean = 0.0094) |
| Refractive Index |
INDEX OF REFRACTION (LIQ): 1.374 |
| Dissociation Constants |
pKa1 = 7.04; pKa2 = 11.96 |
| Kovats Retention Index |
340 338 338 |
| Other Experimental Properties |
Triple Point; 187.62 K; -85.5 °C; -122.0 °F: Absolute density, Gas at 101.325 kPa at 25 °C 1.506 kg/cu m: Relative density, Gas at 101.325 kPa at 25 °C (Air = 1) 1.188: Density, Liquid at Saturation Pressure at -60.3 °C 0.960 kg/l: Critical Temperature 373.56 K; 100.4 °C; 212.7 °F: Critical Volume 2.867 dm3/kg: Critical Density 0.349 kg/dm3: Critical Compressibility Factor 0.283: Latent Heat of Fusion at -85.5 °C 2 376.5 J/mol; 69 741.2 J/kg; 16.67 kcal/kg Thermal Conductivity, Gas at 101.325 kPa at 15.6 °C 0.014 004 W/(m.K); 33.5 x 10-6 cal/(sq cm °C) Solubility In Water at 101.325 kOPa at 25 °C 2.257 cu m/1 cu m water: Index of Refraction, Gas at 101.325 kPa, ND at 25 °C 1.000 584 5: Dielectric Constant; Gas at 0 °C, 101.325 kPa 1.004: Liquid at -78.5 °C 9.05. |
| Chemical Classes |
Toxic Gases & Vapors -> Chemical Asphyxiants |