CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Pale yellow crystals; [CAMEO] Yellowish solid with an odor of soured fruit; mp = 29 deg C; [HSDB] Pure: Yellow to white solid; Impure: Oily brown liquid; [EPA OHM/TADS] |
|---|---|
| Color/Form | YELLOWISH CRYSTALS FROM DILUTE ALCOHOL |
| Odor | ODOR OF SOURED FRUIT |
| Boiling Point | 242 °C @ 760 MM HG |
| Melting Point | 77 °F |
| Solubility | SLIGHTLY SOL IN WATER; FREELY SOL IN ALCOHOL, ETHER, ACETONE, CHLOROFORM, COMMON ORGANIC SOLVENTS; SOL IN PHOSGENE, CHLOROPICRIN, BENZYL CYANIDE |
| Density | 1.539 @ 29 °C/4 °C |
| Vapor Density | 6.8 (AIR= 1) |
| Vapor Pressure | 0.00465 [mmHg] |
| Odor Threshold | LOWEST DETECTABLE LEVEL: 0.09 MG/CU M |
| Other Experimental Properties | MELTS TO AN OILY LIQ JUST ABOVE ROOM TEMP |
| Chemical Classes | Toxic Gases & Vapors -> Tear Gas Agents |
COMPUTED DESCRIPTORS
| Molecular Weight | 196.04 g/mol |
|---|---|
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 194.96836 g/mol |
| Monoisotopic Mass | 194.96836 g/mol |
| Topological Polar Surface Area | 23.8 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 141 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
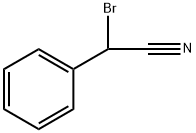
Bromobenzyl cyanide is a colorless organobromide compound. It is slightly soluble in water but readily soluble in organic solvents. Bromobenzyl cyanide is resistant to the action of water and oxidizers; it decomposes upon heating above 120° C and also when exposed to the action of a number of metals, which are thereby intensely corroded. Bromobenzyl cyanide is obtained by the action of sodium cyanide or potassium cyanide on benzyl chloride with subsequent bromination of the benzyl cyanide that has been formed. Bromobenzyl cyanide acts powerfully on the mucous membranes of the eye, causing irritation and heavy lachrymation. It was proposed as a toxic lachrymatory agent at the end of World War I and an irritant gas for law enforcement. It is chemically and biologically similar to Benzyl cyanide. Benzyl cyanide and its derivatives are used in organic synthesis for dyes, perfumes, pesticides, pharmaceuticals, especially penicillin precursors.