5421-46-5
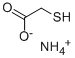
Product Name:
Ammonium thioglycolate
Formula:
C2H7NO2S
Synonyms:
Thioglycolic acid ammonium salt
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Ammonium thioglycolate is a colorless to faint pink liquid with a repulsive, skunk-like odor. (NTP, 1992) |
|---|---|
| Color/Form | Colorless to pale pink liquid |
| Odor | Repulsive odor |
| Solubility | Miscible (NTP, 1992) |
| Density | Specific gravity: 1.205 @ 20 °C |
| Vapor Pressure | 0.0000085 [mmHg] |
| LogP | log Kow = -2.85 /Estimated/ |
| Decomposition | When heated to decomposition it emits very toxic fumes of /nitrogen oxides, sulfur oxides, and ammonia/. |
| pH | pH = 7.0-7.2 |
| Dissociation Constants | pKa1: 3.55 @ 25 °C; pKa2: 10.22 @ 25 °C /Thioglycolic acid/ |
| Other Experimental Properties | Hydroxyl radical reaction rate constant: 3.4X10-11 cu cm/molecule-sec @ 25 °C /Estimated/ |
| Chemical Classes | Other Classes -> Thioglycolates |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H290:Corrosive to Metals H301:Acute toxicity,oral H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
COMPUTED DESCRIPTORS
| Molecular Weight | 109.15 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 109.01974964 g/mol |
| Monoisotopic Mass | 109.01974964 g/mol |
| Topological Polar Surface Area | 42.1 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 37.4 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ammonium thioglycolate is a colorless to faint pink liquid with a repulsive, skunk-like odor. (NTP, 1992)