53112-28-0
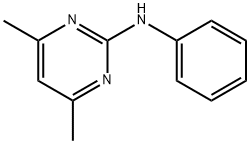
Product Name:
Pyrimethanil
Formula:
C12H13N3
Synonyms:
2-Anilino-4,6-dimethylpyrimidine
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | White solid; [Merck Index] Colorless solid; [HSDB] Faintly yellow powder; [MSDSonline] |
|---|---|
| Color/Form | Colorless crystals |
| Melting Point | 96.3 °C |
| Solubility | In acetone, 389 g/l @ 20 °C; ethyl acetate, 617 g/l @ 20 °C; methanol, 176 g/l @ 20 °C; methylene chloride, 1000 g/l @ 20 °C; n-hexane, 23.7 g/l @ 20 °C; toluene, 412 g/l @ 20 °C. |
| Density | 1.15 g/cu cm @ 20 °C |
| Vapor Pressure | 0.0000165 [mmHg] |
| LogP | log Kow= 2.84 |
| Stability/Shelf Life | Stable in water within the relevant pH range. Stable for 14 days at 54 °C. |
| Ionization Efficiency | Positive |
| Dissociation Constants | pKa (conjugate acid)= 3.52 |
| Collision Cross Section | 142.95 Ų [M+H]+ [CCS Type: TW] |
| Kovats Retention Index | 1762.4 |
| Chemical Classes | Pesticides -> Fungicides |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
COMPUTED DESCRIPTORS
| Molecular Weight | 199.25 g/mol |
|---|---|
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 199.110947427 g/mol |
| Monoisotopic Mass | 199.110947427 g/mol |
| Topological Polar Surface Area | 37.8 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 179 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Pyrimethanil is a member of the class of aminopyrimidines that is N-phenylpyrimidin-2-amine carrying two additional methyl substituents at positions 4 and 6. A fungicide used to control grey mould on fruit, vegetables and ornamentals as well as leaf scab on pome fruit. Also commonly employed to control Botrytis cinerea throughout the winemaking process in grapes, must, fermenting must and wine. It has a role as an aryl hydrocarbon receptor agonist, an environmental contaminant, a xenobiotic and an antifungal agrochemical. It is an aminopyrimidine, a secondary amino compound and an anilinopyrimidine fungicide.