512-04-9
Product Name:
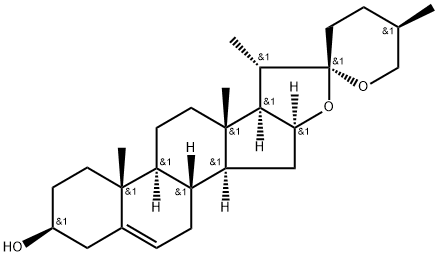
Diosgenin
Formula:
C27H42O3
Synonyms:
(25R)-5-Spirosten-3β-ol;3β-Hydroxy-5-spirostene;3β-hydroxy-5-spirostene; nitogenin;Nitogenin
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Collision Cross Section | 208.4 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
|---|
COMPUTED DESCRIPTORS
| Molecular Weight | 414.6 g/mol |
|---|---|
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 414.31339520 g/mol |
| Monoisotopic Mass | 414.31339520 g/mol |
| Topological Polar Surface Area | 38.7 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 746 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 11 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Diosgenin is a sapogenin that is spirostan which is substituted by a hydroxy group at the 3beta position, contains a double bond at the 5-6 position, and has R- configuration at position 25. A natural product found in Dioscorea (wild yam) species, it is used as the starting point for the commercial synthesis of a number of steroids, including cortisone, pregnenolone and progesterone. It has a role as an apoptosis inducer, an antiviral agent, an antineoplastic agent and a metabolite. It is a 3beta-sterol, a spiroketal, a hexacyclic triterpenoid and a sapogenin. It derives from a hydride of a spirostan.
