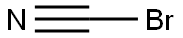CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Cyanogen bromide is a colorless to white crystalline solid with a penetrating odor. It is slightly soluble in water. It is gradually decomposed by water and very rapidly by acids to give off hydrogen bromide, a flammable and poisonous gas. Contamination with many materials can cause rapid decomposition of the material. It is toxic by inhalation of its vapors or by the hydrogen cyanide from decomposition or by ingestion. Toxic oxides of nitrogen are produced in fire involving this material. It is used in gold extraction, to make other chemicals, and as a fumigant. |
|---|---|
| Color/Form | White hygroscopic needles |
| Odor | Penetrating |
| Taste | Bitter |
| Boiling Point | 142 °F at 760 mmHg (EPA, 1998) |
| Melting Point | 126 °F (EPA, 1998) |
| Flash Point | Not Applicable. Not flammable. (USCG, 1999) |
| Solubility | In water, soluble with hydrolysis |
| Density | 2.015 at 68 °F (EPA, 1998) - Denser than water; will sink |
| Vapor Density | 3.62 (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 92 mmHg at 68 °F (EPA, 1998) |
| Stability/Shelf Life | Cyanogen bromide is moderately endothermic ... & shows evidence of instability. |
| Autoignition Temperature | Not Applicable. Not flammable. (USCG, 1999) |
| Decomposition | ... If involved in a fire decomposes to produce toxic gases. |
| Corrosivity | Corrodes most metals |
| Heat of Vaporization | 33.82 kJ/mol |
| Kovats Retention Index | 482 482 |
| Other Experimental Properties | Decomposes in the presence of water |
| Chemical Classes | Toxic Gases & Vapors -> Cyanides |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 105.92 g/mol |
|---|---|
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 104.92141 g/mol |
| Monoisotopic Mass | 104.92141 g/mol |
| Topological Polar Surface Area | 23.8 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 31.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Cyanogen bromide is a colorless to white crystalline solid with a penetrating odor. It is slightly soluble in water. It is gradually decomposed by water and very rapidly by acids to give off hydrogen bromide, a flammable and poisonous gas. Contamination with many materials can cause rapid decomposition of the material. It is toxic by inhalation of its vapors or by the hydrogen cyanide from decomposition or by ingestion. Toxic oxides of nitrogen are produced in fire involving this material. It is used in gold extraction, to make other chemicals, and as a fumigant.