CHEMICAL AND PHYSICAL PROPERTIES
| Collision Cross Section | 165.1 Ų [M+Na]+ 151.68 Ų [M+H]+ |
|---|---|
| Kovats Retention Index | 1847.7 |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H330:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
COMPUTED DESCRIPTORS
| Molecular Weight | 255.74 g/mol |
|---|---|
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | 255.1026065 g/mol |
| Monoisotopic Mass | 255.1026065 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 238 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
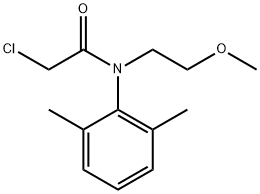
description
Dimethachlor is an organochlorine compound that is 2-chloroacetamide substituted by a 2-methoxyethyl and a 2,6-dimethylphenyl group at the nitrogen atom. It has a role as a xenobiotic, an environmental contaminant and a herbicide. It is an aromatic amide, an ether and an organochlorine compound.