CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Colorless solid; Insoluble in water; [ICSC] Pale yellow powder; Insoluble in water; [MSDSonline] |
|---|---|
| Color/Form | COLORLESS CRYSTALLINE POWDER |
| Odor | Faint aromatic odor |
| Melting Point | 66-67 °C |
| Solubility | Insoluble in water but soluble in organic solvents as follows: acetone 78%; ethanol 11%; isophorone 57%; kerosene 10%; xylene 70% |
| Density | 1.2307 @ 20 °C/4 °C |
| Vapor Pressure | 0.00000032 [mmHg] |
| Stability/Shelf Life | ... Unstable in alkali & concentrated acids, suffers slight hydrolysis on long contact with water & is slowly decomposed by ultra violet light. |
| Kovats Retention Index | 2200 2195.3 2204.3 |
| Other Experimental Properties | VAPOR PRESSURE: 1X10-4 MM HG @ 60 °C |
| Chemical Classes | Pesticides -> Other Insecticides |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 322.31 g/mol |
|---|---|
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 322.11648630 g/mol |
| Monoisotopic Mass | 322.11648630 g/mol |
| Topological Polar Surface Area | 118 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 489 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
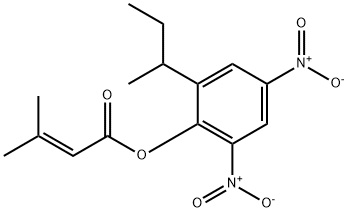
2-sec-butyl-4,6-dinitrophenyl 3-methylbut-2-enoate is an enoate ester obtained by formal condensation of the carboxy group of 3,3-dimethylacrylic acid with the phenolic hydroxy group of dinoseb. It is a C-nitro compound and an enoate ester. It is functionally related to a dinoseb.