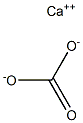
471-34-1
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | White hexagonal crystals or powder (Calcite); white orthrombic crystals or powder (Argonite); colorless hexagonal crystals (vaterite) |
|---|---|
| Odor | Odorless |
| Taste | Chalky taste |
| Boiling Point | Decomposes (NIOSH, 2023) |
| Melting Point | 1517 to 2442 °F (Decomposes) (NIOSH, 2023) |
| Solubility | 0.001 % (NIOSH, 2023) |
| Density | 2.7 to 2.95 (NIOSH, 2023) |
| Vapor Pressure | 0 mmHg (approx) (NIOSH, 2023) |
| Stability/Shelf Life | Indefinite shelflife. |
| Decomposition | When heated to decomposition it emits acrid smoke and irritating vapors. |
| Corrosivity | Non-corrosive |
| pH | pH = 8 to 9 |
| Refractive Index | Index of Refraction: 1.7216 (300 nm); 1.6584 (589 nm); 1.6503 (750 nm) /Calcite/ |
| Other Experimental Properties | Very flowable ... surface treated with fatty acids, hydrophobic |
| Chemical Classes | Mineral Dusts -> Other Mineral Dusts |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 100.09 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 99.9473347 g/mol |
| Monoisotopic Mass | 99.9473347 g/mol |
| Topological Polar Surface Area | 63.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Calcium carbonate, chemically known as CaCO₃, is a common inorganic salt. It is one of the most abundant compounds found in the Earth's crust and can be found in everyday foods such as eggs and oyster shells, crustacean exoskeletons, and dark leafy greens like broccoli and kale. CaCO₃ is used therapeutically as a food additive, dietary supplement, antacid, and phosphate binder.
Uses
Calcium carbonate is a commonly occuring compound used as a buffer and an antacid. It can treats heartburn, indigestion, upset stomach or other symptoms caused by too much stomach acid.
Calcium carbonate is an inorganic salt primarily used to manage and treat low calcium conditions, GERD, CKD, and other indicated conditions.
Calcium carbonate is classified as a calcium supplement, antacid, and phosphate binder.
