CHEMICAL AND PHYSICAL PROPERTIES
| Solubility | Soluble |
|---|
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 616.7 g/mol |
|---|---|
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 8 |
| Exact Mass | 616.34721450 g/mol |
| Monoisotopic Mass | 616.34721450 g/mol |
| Topological Polar Surface Area | 170 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 1230 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
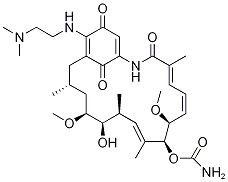
PRODUCT INTRODUCTION
description
Alvespimycin is a 19-membered macrocyle that is geldanamycin in which the methoxy group attached to the benzoquinone moiety has been replaced by a 2-(N,N-dimethylamino)ethylamino group. It has a role as a Hsp90 inhibitor. It is a secondary amino compound, a tertiary amino compound, an ansamycin, a member of 1,4-benzoquinones and a carbamate ester. It is functionally related to a geldanamycin.