353-42-4
Product Name:
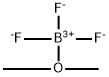
Boron trifluoride dimethyl etherate
Formula:
C2H6BF3O
Synonyms:
(Dimethyl ether)trifluoroboron
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Boron trifluoride dimethyl etherate appears as a fuming liquid. Corrosive to skin, eyes and mucous membranes. May be toxic by inhalation. Used as a catalyst in chemical reactions. |
|---|---|
| Color/Form | Liquid |
| Boiling Point | 258.8 °F at 760 mmHg (EPA, 1998) |
| Melting Point | -14 °C |
| Flash Point | 62 °C |
| Density | 1.239 |
| Vapor Pressure | 17.3 [mmHg] |
| Decomposition | When heated to decomposition it emits toxic fumes of /hydrogen fluoride/. |
| Corrosivity | Corrosive |
| Other Experimental Properties | Reacts violetnly with water |
| Chemical Classes | Metals -> Metalloid Compounds (Boron) |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H302:Acute toxicity,oral H314:Skin corrosion/irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 113.88 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 114.0463795 g/mol |
| Monoisotopic Mass | 114.0463795 g/mol |
| Topological Polar Surface Area | 9.2 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 24.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Boron trifluoride dimethyl etherate appears as a fuming liquid. Corrosive to skin, eyes and mucous membranes. May be toxic by inhalation. Used as a catalyst in chemical reactions.