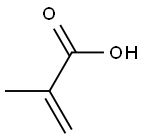
| Physical Description |
Methacrylic acid appears as a clear colorless liquid (or low-melting solid) with a pungent odor. Corrosive to metals and tissue. Flash point 170 °F. Melting point 61 °F. May polymerize exothermically if heated or contaminated. If the polymerization takes place inside a container, the container may rupture violently. Less dense than water. Vapors heavier than air. Used to make plastics. |
| Color/Form |
Clear colorless liquid or colorless crystals |
| Odor |
Acrid, repulsive odor |
| Boiling Point |
325 °F at 760 mmHg (NTP, 1992) |
| Melting Point |
61 °F (NTP, 1992) |
| Flash Point |
170 °F (NTP, 1992) |
| Solubility |
greater than or equal to 100 mg/mL at 63 °F (NTP, 1992) |
| Density |
1.015 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density |
2.97 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure |
0.65 mmHg at 68 °F ; 1 mmHg at 77 °F (NTP, 1992) |
| LogP |
log Kow = 0.93 |
| Henry's Law Constant |
Henry's Law constant = 3.9X10-7 atm-cu m/mole at 25 °C |
| Stability/Shelf Life |
Acrylic acid and methacrylic acid readily polymerize in the presence of light, heat and oxygen, and also under the action of oxidizing agents such as peroxides. |
| Autoignition Temperature |
752 °F (USCG, 1999) |
| Decomposition |
When heated to decomposition it emits acrid smoke and irritating fumes. |
| Viscosity |
1.38 mPa.s at 24 °C |
| Corrosivity |
Forms corrosive liquid at melting point |
| Heat of Vaporization |
0.418 kJ/mol at 101.3 kPa (760 mm Hg) |
| Surface Tension |
0.0265 N/m |
| Polymerization |
POLYMERIZES EASILY, ESP ON HEATING OR IN PRESENCE OF TRACES OF HYDROCHLORIC ACID |
| Refractive Index |
Index of refraction: 1.43143 at 20 °C/D |
| Dissociation Constants |
pKa = 4.65 |
| Kovats Retention Index |
711 |
| Other Experimental Properties |
Specific heat capacity: 2.1 J/g K; critical volume: 270 cu cm/mol |
| Chemical Classes |
Other Classes -> Organic Acids |