23491-45-4
Product Name:
Hoechst 33258
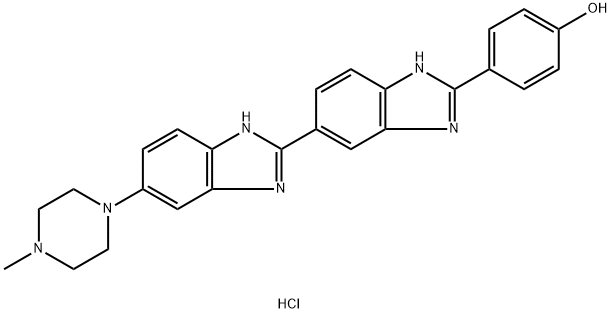
Formula:
C25H25ClN6O
Synonyms:
2-[2-(4-Hydroxyphenyl)-6-benzimidazoyl]-6-(1-methyl-4-piperazyl)-benzimidazole, 3HCl, HOE 33258;Bisbenzimide H 33258;Bisbenzimide H 33258 Fluorochrome, Trihydrochloride - CAS 23491-45-4 - Calbiochem;bisBenzimide H 33258 solution
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Yellow-green powder; [Acros Organics MSDS] |
|---|---|
| Solubility | DMSO 240 - 300 (mg/mL) |
| Stability/Shelf Life | Bulk: Under ordinary laboratory illumination, the material slowly decomposes. At the end of four weeks at room temperature in a capped glass vial, 3% decomposition has occurred (HPLC). In a similar container but maintained at 45 °C and in darkness, the subject sample was stable for at least four weeks (HPLC). Solution: A solution of the sample (1 mg/mL) is stable in water for at least 24 hours at room temperature under normal laboratory illumination. |
| Chemical Classes | Dyes -> Other Dyes |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 533.9 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 532.131193 g/mol |
| Monoisotopic Mass | 532.131193 g/mol |
| Topological Polar Surface Area | 84.1 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 634 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 4 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
A benzimidazole antifilarial agent; it is fluorescent when it binds to certain nucleotides in DNA, thus providing a tool for the study of DNA replication; it also interferes with mitosis.