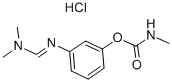
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Formetanate hydrochloride is a white powder with a faint odor. Used as a plant insecticide, acaricide, and miticide. (EPA, 1998) |
|---|---|
| Color/Form | COLORLESS CRYSTALS |
| Odor | FAINT ODOR |
| Melting Point | 392 to 396 °F decomposes (EPA, 1998) |
| Solubility | YELLOW CRYSTALLINE SOLID; SOLUBILITY: LESS THAN 1 G/L WATER; ABOUT 100 G/L ACETONE, CHLOROFORM; GREATER THAN 200 G/L METHANOL /FORMETANATE/ |
| Vapor Pressure | 2e-08 mmHg (EPA, 1998) |
| Stability/Shelf Life | NON-VOLATILE |
| Other Experimental Properties | DECOMP @ 200-202 °C |
| Chemical Classes | Pesticides -> Carbamate Insecticides |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
COMPUTED DESCRIPTORS
| Molecular Weight | 257.72 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 257.0931045 g/mol |
| Monoisotopic Mass | 257.0931045 g/mol |
| Topological Polar Surface Area | 53.9 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 254 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Formetanate hydrochloride is a white powder with a faint odor. Used as a plant insecticide, acaricide, and miticide. (EPA, 1998)