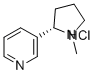
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Nicotine hydrochloride appears as a white crystalline solid. Combustible, although ignition may be difficult. Toxic by inhalation (dust, etc.) and skin absorption. Produces toxic oxides of nitrogen during combustion. |
|---|---|
| Color/Form | Colorless oil |
| Solubility | All salts are soluble in water, alcohol, and ether /Nicotine salts/ |
| Density | DENSITY 1.0337 /D-FORM/ |
| Optical Rotation | Crystals. Delliquescent. Specific optical rotation = +104 deg at 20 °C/D ( p = 10) /l-Form/ |
| Decomposition | When heated to decomposition it emits very toxic fumes of /hydrogen chloride, nitrogen oxides and carbon monoxide./ |
COMPUTED DESCRIPTORS
| Molecular Weight | 198.69 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 198.0923762 g/mol |
| Monoisotopic Mass | 198.0923762 g/mol |
| Topological Polar Surface Area | 16.1 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 147 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Nicotine hydrochloride appears as a white crystalline solid. Combustible, although ignition may be difficult. Toxic by inhalation (dust, etc.) and skin absorption. Produces toxic oxides of nitrogen during combustion.