213464-77-8
Product Name:
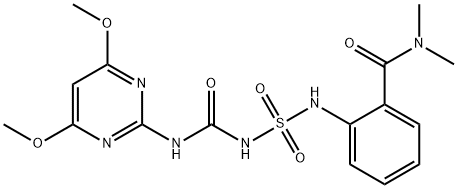
Orthosulfamuron
Formula:
C16H20N6O6S
Synonyms:
1-(4,6-Dimethoxy-2-pyrimidinyl)-3-[2-(dimethylcarbamoyl)phenylsulfamoyl]urea
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | Very fine white powder with a tendency to form clumps |
|---|---|
| Boiling Point | Decomposes before boiling |
| Melting Point | 157 °C |
| Solubility | In n-heptane 0.23, xylene 129.8 (both in mg/L, 20 °C). In acetone 19.5, ethyl acetate 3.3, 1,2-dichloromethane 56.0, methanol 8.3 ( all in g/L, 20 °C) |
| Density | 1.48 at 22.0 °C |
| Vapor Pressure | 8.4X10-7 mm Hg at 20 °C at 25 °C |
| LogP | log Kow = 1.31 (pH 7); 2.02 (pH 4); <0.3 (pH 9) |
COMPUTED DESCRIPTORS
| Molecular Weight | 424.4 g/mol |
|---|---|
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 424.11650355 g/mol |
| Monoisotopic Mass | 424.11650355 g/mol |
| Topological Polar Surface Area | 160 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 657 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Orthosulfamuron is an N-sulfonylurea and sulfuric amide resulting from the formal condensation of sulfuric acid with the primary amino group of 1-(4,6-dimethoxypyrimidin-2-yl)urea and with the primary amino group of 2-amino-N,N-dimethylbenzamide (1 mol eq. of each). An acetolactate synthase inhibitor, it is used as a pre- and post-emergent herbicide for the treatment of grasses and broad-leaved weeds in rice crops. It has a role as an EC 2.2.1.6 (acetolactate synthase) inhibitor and a herbicide. It is a tertiary carboxamide, a sulfuric amide, a N-sulfonylurea and an aromatic ether.