CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | White solid; [Merck Index] White crystalline solid; [MSDSonline] |
|---|---|
| Color/Form | White crystalline solid |
| Melting Point | 116 °C |
| Solubility | Solubility (g/L, 20 °C): hexane 0.057, n-octanol 1.4, methanol 20, toluene 55, acetone 86, ethyl acetate 130, acetonitrile 340, dichloromethane 400 |
| Density | 1.33 |
| Vapor Pressure | 8.3X10-13 mm Hg at 25 °C |
| LogP | log Kow = 2.50 at 20 °C |
| Stability/Shelf Life | Photostable. |
| Decomposition | When heated to decomposition it emits toxic vapors of /nitrogen oxides/. |
| Ionization Efficiency | Positive |
| Collision Cross Section | 195.75 Ų [M+H]+ [CCS Type: TW] |
| Kovats Retention Index | 3083 3047.2 3076.2 |
| Other Experimental Properties | Color: Beige solid /Heritage fungicide/ |
| Chemical Classes | Pesticides -> Fungicides |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H331:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
COMPUTED DESCRIPTORS
| Molecular Weight | 403.4 g/mol |
|---|---|
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 8 |
| Exact Mass | 403.11682065 g/mol |
| Monoisotopic Mass | 403.11682065 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 646 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
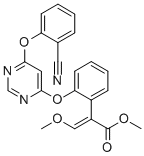
Azoxystrobin is an aryloxypyrimidine having a 4,6-diphenoxypyrimidine skeleton in which one of the phenyl rings is cyano-substituted at C-2 and the other carries a 2-methoxy-1-(methoxycarbonyl)vinyl substituent, also at C-2. An inhibitor of mitochondrial respiration by blocking electron transfer between cytochromes b and c1, it is used widely as a fungicide in agriculture. It has a role as a mitochondrial cytochrome-bc1 complex inhibitor, a xenobiotic, an environmental contaminant, an antifungal agrochemical and a quinone outside inhibitor. It is a nitrile, an aryloxypyrimidine, an enoate ester, an enol ether, a methyl ester and a methoxyacrylate strobilurin antifungal agent.